Story at a Glance: •The FDA was established in 1906 in response to public concern over unsafe food and drugs, such as spoiled food and counterfeit products. However, food industry lobbyists gradually gained influence, leading to the removal of the agency's original leader. As a result, numerous harmful food additives were granted "generally recognized as safe" (GRAS) status and continue to be used today. •In 1962, the FDA was given broad powers to oversee drug safety following the thalidomide incident. Unfortunately, the new regulations created strict standards for drug efficacy that were often selectively enforced, benefiting the pharmaceutical industry. Unfortunately, the FDA increasingly targeted natural therapies, which led to many being erased from history. •Despite numerous attempts to reform the agency, issues of inefficiency and bias within the FDA persist. This article examines these challenges and suggests potential reforms to improve the agency’s role in safeguarding public health. For most of my life, I’ve observed the FDA belligerently suppress natural treatments and any unorthodox therapy which threatens the medical monopoly while simultaneously railroading through a variety of unsafe and ineffective drugs regardless of how much public protest the agency meets. Consider this 2004 Senate testimony by the FDA scientist who got Vioxx banned that accurately described exactly what would come to pass with the COVID vaccines two decades later: As such, I do not hold the FDA in a positive light, especially given that during COVID-19, I (like many others) spent hundreds of hours trying to get the agency to allow the limited use of off-patent therapeutics for COVID-19—all of which ultimately went nowhere due to the unjustifiable roadblocks the agency kept putting up. Over the past year, especially since Trump's election, I've received many questions about FDA reform. To address the issue properly, I’ve carefully examined both sides. In medicine, "sensitivity" refers to a test’s ability to correctly identify those who have a condition (e.g., detecting an infection), while "specificity" measures how well the test avoids false positives (i.e., correctly identifying those who don’t have the condition). The challenge is that improving one often reduces the other. For example, increasing the PCR cycle threshold in COVID tests made it more likely to detect infections (higher sensitivity), but also increased false positives (lower specificity). This trade-off leads to problems, like breast cancer screenings, where high sensitivity can result in false positives and unnecessary “treatments” for women who don’t actually have cancer. The FDA faces a similar challenge: it must prevent harmful foods and drugs from reaching the market while ensuring useful products aren’t blocked. Though this seems straightforward, it’s incredibly difficult, and the FDA has often failed at both, even with leadership dedicated to public health. Crime Against the Food LawIn the late 1800s, food producers were selling adulterated products, and pharmaceutical companies peddled medicines with secret ingredients like opium and alcohol. Public outrage grew, especially after exposés like Upton Sinclair’s The Jungle, which helped spark the 1906 Pure Food and Drug Act. This law gave the Bureau of Chemistry the power to ensure accurate labeling and prevent harmful additives in food. The director of the Bureau of Chemistry (and thus the first head of the FDA), Harvey Wiley conducted tests on food additives, proving they made healthy volunteers sick. While the public and many scientists supported his findings, the food industry fought back with powerful lobbyists and legal tactics. Note: the additives Wiley scrutinized were boric acid and borax, salicylic acid (aspirin) and salicylates, benzoic acid and benzoates, sulfur dioxide and sulfites, formaldehyde, sulfate of copper (used to green produce), and saltpeter (nitrates). Gradually,
the food industry hijacked the presidency, and in 1912, Wiley resigned,
realizing he could achieve more for America’s health as a private
citizen than within the government. Those tactics also highlight a key point Wiley made—the only way to create change in this industry is to coax the public at large to demand it, as the moment you rely upon the members of the government to fix it, lobbyists will crush those efforts. Generally Recognized as “Safe”Many food additives are "generally recognized as safe" (GRAS), meaning they’re widely used without regulation. Wiley faced two major issues: food industry counterfeiting and harmful additives. The industry often faked products to cut costs, like selling grain alcohol as whiskey or using polluted waters to enlarge oysters. Despite evidence of harm, the food industry claimed these additives were essential for production, even though competitors showed higher-quality (and ultimately more profitable) products could be made without them. Wiley also warned that chronic exposure to additives could cause long-term health issues, such as organ damage and aging. Sadly, his concerns were ignored as industry influence grew and he was unable to ban them—rather they were eventually reclassified as “generally recognized as safe.” As a result, these "safe" additives have contributed to widespread chronic illness in society. Note: those additives included sodium benzoate, sulfur dioxide, alum (potassium aluminum sulfate), sulfur dioxide, saccharin, modified corn sugars, saccharin, and nitrogen bleached flour—many of which were linked to cancer. Sadly, since 2000, nearly 99 percent of new food chemicals added to the food supply chain have exploited the GRAS loophole. I believe the widespread use of aluminum in processed foods is particularly detrimental (due to it greatly impairing the physiologic zeta potential and causing micro-clotting throughout the body), and provides a key explanation for why you often see certain rapid improvements in individuals once they stop eating processed foods and their additives. The Kefauver–Harris AmendmentIn the years that followed Wiley’s departure, the handicapping of the FDA continued. As such, the FDA agent assigned to the morning sickness drug thalidomide could only stall but not reject it—a tactic that prevented catastrophic birth defects across America. A 1962 amendment was then passed, giving the FDA the power to block unsafe drugs. This law gave the FDA excessive power, slowing drug approval and causing mismanagement. It also required "well-controlled" trials for drug approval, which the FDA defined as expensive double-blind randomized controlled trials (RCTs). This:
Because the FDA had rapidly expanded in numerous directions it was not prepared for, it subsequently frequently failed to fulfill its primary responsibilities (e.g., taking something harmful off the market), and it simultaneously took things away Americans actually wanted. This in turn led to numerous committees investigating the FDA (e.g., Commissioner Lay’s Kinslow report of his agency’s serious shortcomings) and key officials with integrity like Lay being kicked out, all of which were encapsulated a series of scathing articles that were published by the New York Times in 1977. In my eyes, the most important thing about this period of FDA reforms was that the FDA was the most complained about agency in the government. Congress made numerous attempts to fix it (as did ethical FDA officials)—but nothing was ever solved. The DMSO SagaOver
the last seven months, I’ve begun exploring a remarkable forgotten side
of medicine—DMSO. This simple and freely available natural chemical is
incredibly effective at treating a variety of (often “incurable”)
conditions, including many that are otherwise impossible to treat
including: Likewise, since publicizing this research, I’ve received over two thousand reports from readers who then took it and had almost unbelievable results that precisely match what many reported in the 1960s and 1970s. This all raises a simple question. How is it that no one knows about DMSO or that an agent that could dramatically reduce the need for opioids or prevent millions with stroke and spinal cord injury from having a life of disability never saw the light of day? That’s because as DMSO rapidly spread across America in the 1960s, the FDA reversed its initial positive stance, declaring DMSO dangerous without evidence. This pivot was initially prompted by the FDA not wanting to have to process a flood of new drug applications, and then evolved into being done to protect the status quo and to justify the FDA’s newfound police powers. Despite extensive safety studies showing DMSO posed no risk to humans and numerous Congressional hearing being held to legalize DMSO, for decades, the FDA continued to demonize it, claiming a lack of evidence for efficacy (as DMSO’s characteristic effects make blinded trials with it impossible). Because of this, DMSO only became available decades later after the public got fed up with the FDA targeting natural medicines and the 1994 Dietary Supplement Health and Education Act was enacted (which removed the FDA’s ability to regulate natural medicines). The FDA’s War Against Natural MedicineShortly before the election, RFK Jr. gave what I considered to be one of the most important statements in the entire campaign: This tweet touched upon the fact that for decades the American Medical Association has done everything it can to remove life-changing natural therapies from the market that compete with the medical monopoly (e.g., ultraviolet blood irradiation and alternative cancer cures) and the FDA has followed in their footsteps. For example: GHB: In the 1990s, a life-changing natural sleep aid spread across America that safely cured insomnia—so the FDA banned it. In contrast, sleeping pills block restorative sleep (which is critical for health) and make you 2-5X more likely to die. Psychedelic Therapy:
MDMA-assisted psychotherapy is the only existing effective treatment
for veterans with PTSD, but the FDA still blocked its approval despite compelling “uncontrolled” clinical trials (as it’s impossible to have blinded psychedelic sessions). Veterans hence often seek treatment abroad due to FDA restrictions. Umbilical Cord Blood Stem Cells: FDA regulations under Biden and Peter Marks made it difficult to offer this life-changing therapy and shut down the companies who provided it. Sunlight and Health: The dermatology industry has deceptively demonized sunlight as a cause of deadly skin cancers, overlooking its importance for overall health and cancer prevention, with studies showing those who avoid sunlight are 60-130% more likely to die. Raw Milk:
The FDA has continually targeted raw milk sellers, despite widespread
demand for it, no evidence linking it to an increased risk of microbial
illness and growing evidence that pasteurization destroys critical
nutrients and creates allergens (discussed further here). Vaccine CoverupsMany have been horrified to learn that the FDA and CDC systematically ignored every possible sign the COVID vaccines were dangerous as they pushed it on more and more people (e.g., recently leaked recordings show how stubbornly the head of FDA’s vaccine division refused to acknowledge any of the evidence brought forward by a group of permanently injured vaccine recipients). This severe betrayal of trust from our authorities thus made many ask, “How could this have happened?” In truth, this did not come out of nowhere. Rather it was simply the subsequent escalation of a longstanding tendency by the government to push vaccines they knew were unsafe and ineffective to market. Vaccine Coverups and Failures by the FDA and CDC:
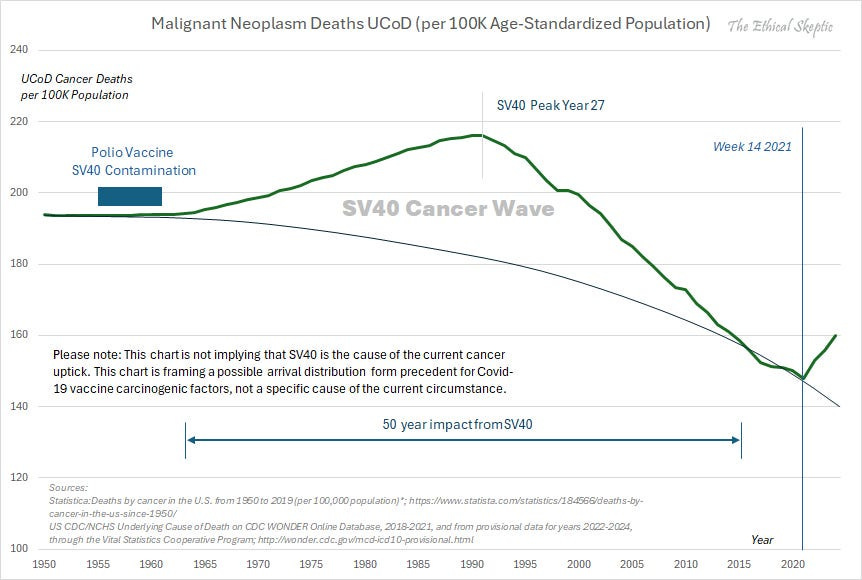
Note: to produce the emergency COVID vaccines at scale, a novel manufacturing process was used, which caused them to be contaminated with dangerous DNA-altering bacterial plasmids that contained part of the SV-40 virus.
Other Disastrous Drug Approvals:
In summary, the FDA has an established history of approving unsafe and ineffective vaccines and drugs, often suppressing evidence and protecting corporate interests over public safety. ConclusionAt this point, I’ve seen a variety of proposals put forward to fix the FDA, which alternate between reforming the agency and scrapping it entirely. In my eyes, the core dilemmas are:
Proposed solutions for restructuring the FDA include:
Previously, implementing ideas like these was impossible, but now that platforms like Twitter (𝕏) have broken the mass media’s stranglehold on democracy and allowed leaders who want to change things to make things better, I believe it can happen (e.g., Secretary Kennedy recently moved to close the GRAS loophole and as shown above, Commissioner Makary just ended pharmaceutical representatives being on the panels which vote to approve their drugs). However, as Wiley presciently warned, that can only happen if the public becomes actively involved—something the MAHA movement (and each of you) now makes possible! Author’s note: This is an abridged version of an article about the FDA’s unconscionable war against DMSO (which set the agency’s behavior for decades to come), an article about the FDA’s past vaccine disasters and a longer article which goes into greater detail on the points mentioned here along with other promising therapies the FDA has blacklisted (which can be read here). To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here. You're currently a free subscriber to The Forgotten Side of Medicine. For the full experience, upgrade your subscription. |





No comments:
Post a Comment