Report 75: mRNA COVID “Vaccines” Have Created a New Class of Multi-Organ/System Disease: “CoVax Disease.” Children from Conception on Suffer Its Devastating Effects. – Histopathology Series – Part 4d
This report is Part 4d in a series. It follows Part 1, “Report 56: Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus;” Part 2, “Report 58: Part 2 – “Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus;” and Part 3, “Report 61: Part 3 –Ute Krüger, MD, Breast Cancer Specialist, Reveals Increase in Cancers and Occurrences of ‘Turbo Cancers’ Following Genetic Therapy ‘Vaccines’,” Part 4a,“Report 64: Part 4a – Re-Humanizing Data Using ‘Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series;“ Part 4b, “Part 4b – Rhabdomyolysis – a.k.a., “Jellied Muscle” – After mRNA Gene Therapy Injections;” and Part 4c, “Report 70: Histopathology Series Part 4c – Autoimmunity: A Principal Pathological Mechanism of COVD-19 Gene Therapy Harm (CoVax Diseases) and a Central Flaw in the LNP/mRNA Platform.”
Summary
Pfizer Report 70 introduced CoVax Disease, a new class of illness that has arisen from the use of mRNA COVID vaccines. CoVax Disease encompasses multiple pathological mechanisms, presents with multi-organ/system involvement, and affects all age groups. This article will focus on the very negative medical impacts on children’s health after receiving mRNA vaccines. Additionally, sex-related effects will be presented.
COVID-19 was a disease primarily of older people and those with comorbidities. Unfortunately, the experimental gene therapy products known as COVID “vaccines” have devastating effects in young, healthy people of both sexes and all ages from conception upward through the age brackets.
Young men have more myo/pericardial disease and more fatalities. Females have more disability and more adverse events overall. As the Cho et al. and Barmada et al. studies document, long-term cardiac disease is one outcome from these drugs.
Disease categories associated with gene therapy products are diverse, are unusual in many cases, and can be unusually severe as in MIS-C, sudden death/cardiac arrest, stroke, and turbo cancer, as illustrated by the intracardiac epithelioid sarcoma case reviewed earlier in this report. Children are not exempt from these illnesses.
Causation is a complicated topic. However, there is ample support presented herein for concluding that some or many of the medical conditions that arise following an injection of C19 gene therapy products were caused by them.
I. Introduction
Since the early medical papers emerging from China in January 2020, it was apparent that the SARS-CoV-2 (SC2) virus preferentially produced disease, COVID-19 (C19), in the elderly with comorbidities. (doi:10.1001/jama.2020.1585) Some younger people with comorbidities also were at risk but the risk for severe disease and death in the general population was low. (https://doi.org/10.1016/S0140-6736(20)30211-7)
Lipid-nanoparticle coated mRNA drugs (LNP/mRNA) were developed to prevent C19 disease. These drugs along with the adenovirus-vectored products will be referred to collectively as gene therapy products (GTPs). Pfizer Confidential 5.3.6 (https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf) documented COVID-19 as an Adverse Event following inoculation with Pfizer’s BNT162b2 gene therapy product as well as over 140,000 additional adverse events reported in the first ten to twelve weeks. (https://dailyclout.io/pfizer-evidence-so-far-coverups-heart-damage-and-more/)
The object of this paper is to review Adverse Event reports following LNP/mRNA gene therapy treatments pertinent to differential age and sex reporting with specific coverage of the zero- to 18-year-old demographic.
Specific clinical features of medical conditions afflicting America’s youth following injection of Spike-mediated genetic treatment will be discussed. The article will conclude by reviewing the time course of Vaccine Adverse Event Reporting System (VAERS) event reporting relative to United States (U.S.) dosing data. VAERS reports are for the U.S. and its Territories unless otherwise specified.
The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) maintain VAERS, which is a public resource. (https://wonder.cdc.gov/controller/datarequest/D8) Open VAERS harvests data from VAERS in specific categories and is useful for summary data. (https://openvaers.com/covid-data)
In addition to VAERS and Open VAERS, this article will make use of case and small series reports from the medical literature, the FDA-released Pfizer Confidential Documents archive (https://phmpt.org/pfizer-16-plus-documents/), and reporting from individuals including William Makis, M.D., Professor Mark Crispin Miller, Ed Dowd and his partners, Jessica Rose, and others.
As a caution, the limitations of VAERS are spelled out on the CDC website:

Reports are not detailed enough for many determinations (1), but the utility of the system is as an early warning system (2). Much of the VAERS system activity is invisible to the user, such as what activity occurs from the time a report is filed with the CDC to when it gets posted and after its initial posting. The numbers that are given in response to queries change over time as the system is updated at least every week. Follow-up data are obtained but not posted.
Events vary in detail from diagnosis only to detailed reporting of medical data. Some categories return cryptic data like “No Adverse Event,” of which there were 32,072 reports for C19 products from 2020 through mid-June 2023 for all ages with 1,003 associated events including 13 deaths.
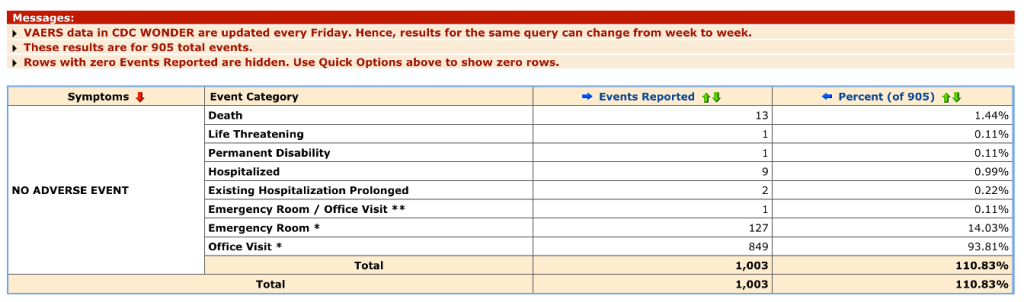
Here is the information of one fatal case so designated. VAERS ID 1205421 was a 61-year-old male who died less than two days after receiving his second dose of mRNA1273 (Moderna). Someone made the effort to file the report but with no details about this man’s death.

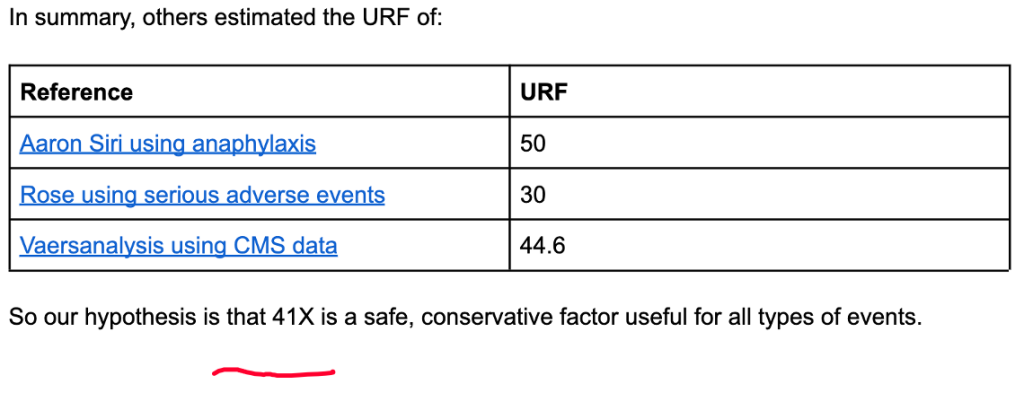
VAERS numbers are generally considered to represent only a fraction of the actual prevalence of the medical conditions reported and for this reason attempts have been made to estimate the true number by estimating what is called the Under Reporting Factor (URF). True prevalence data are hard to find.
Steve Kirsch, Jessica Rose, and Mathew Crawford performed detailed calculations of URF concluding that an URF of 41 was justified. (https://www.skirsch.com/covid/Deaths.pdf, https://jessicar.substack.com/p/the-under-reporting-factor-in-vaers)
II. C19 Gene Therapy: Age and Sex Effects
A. BNT162b2 and mRNA1273 (LNP/mRNA) Approval Schedule
For reference, the following dates are noted:
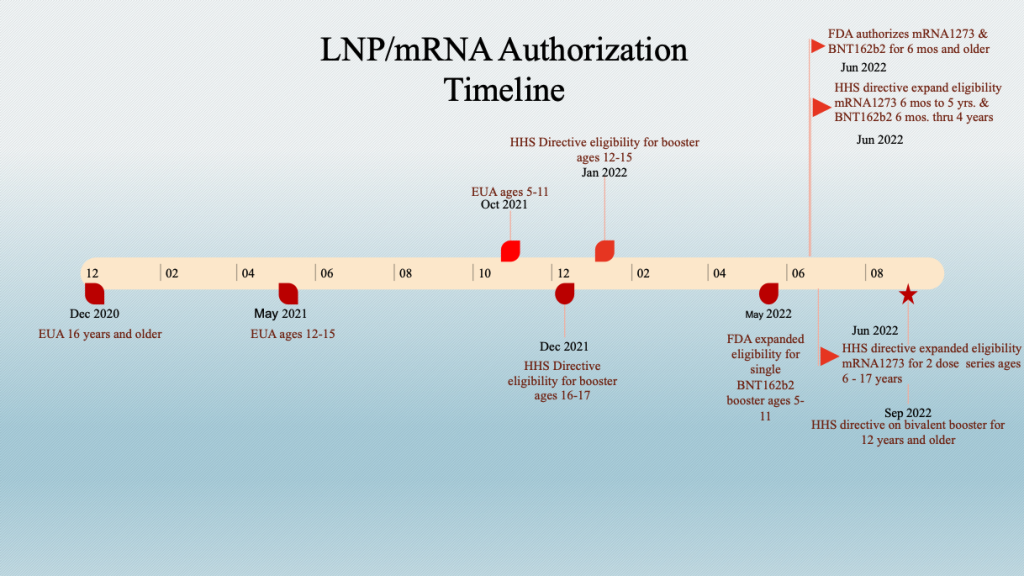
The staggered inoculation of age groups should be kept in mind when looking at data sets that combine age groups. This stagger adds an element of complexity when time series analyses are conducted across age groups.
Example: The age brackets in VAERS do not match the age brackets used for the “vaccine” dosing schedule. However, the six-month-old to 5-year-old group has bracketing close to the corresponding dosing brackets.
Five-year-olds became eligible to receive BNT162b2 on October 29, 2021, and both mRNA drugs were released for six-month-olds to four-year-olds for BNT162b2 and five-year-olds for mRNA1273 on June 18, 2022. The histogram below is a plot of adverse events according to the inoculation schedule showing a spike in event reports following the two authorization dates.
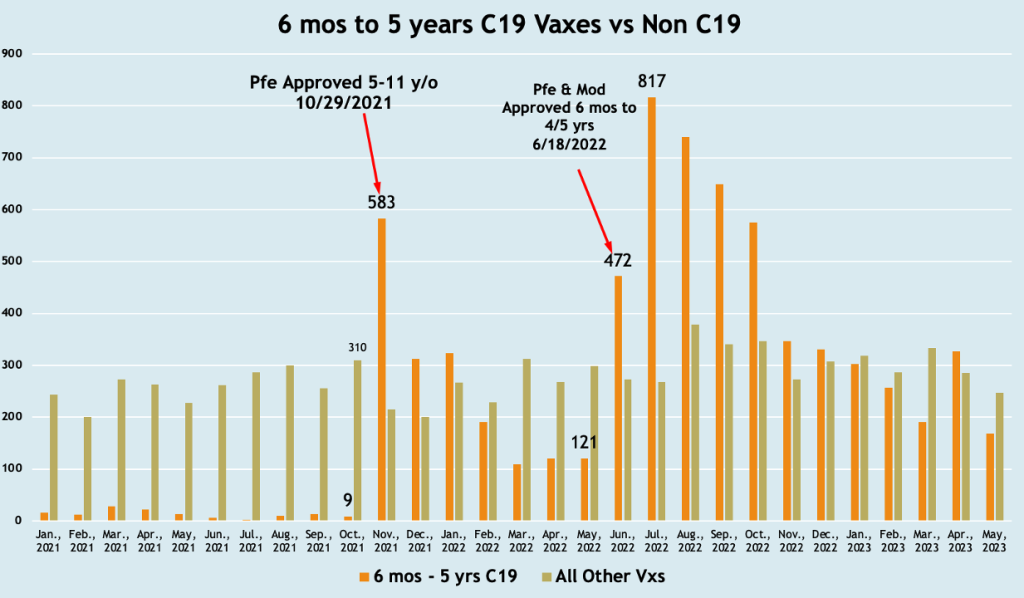
VAERS 6/11/2023
Almost immediately adverse event reporting in VAERS jumped from nine events to 583 events following the October 21, 2021, authorization and from 121 events (full month before) to 817 events (full month afterward). June was a transition month.
B. VAERS Reports: Age and Sex Differences
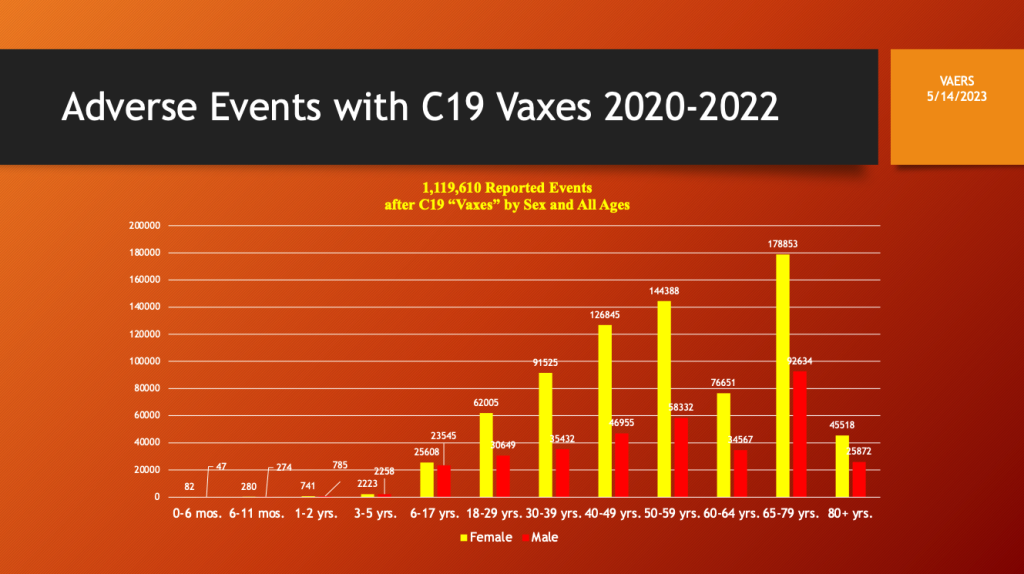
VAERS adverse event reporting was both age and sex dependent with frequency increasing with age. Dosing by age bracket or preferably by year age would be helpful if it were available.
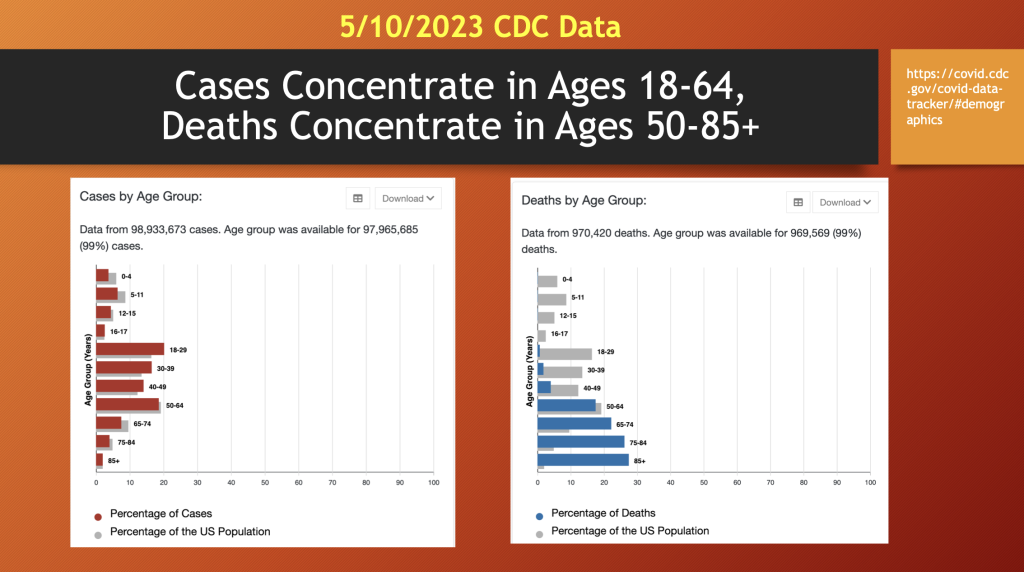
Looking at C19 statistics from the CDC, an interesting pattern emerges with C19 cases concentrated in ages 18 to 64 (46 years), while C19 deaths were more prevalent in the age 50 to 85+ bracket (~35 years), above. Like the early reports, these data identify the primary risk factor for mortality with C19 is age along with co-morbidity. The morbidity and mortality from C19 gene therapy products (GTPs) has more representation in the lower age brackets than C19.
C. VAERS Events: 29 Years of Age and Younger, Menarche
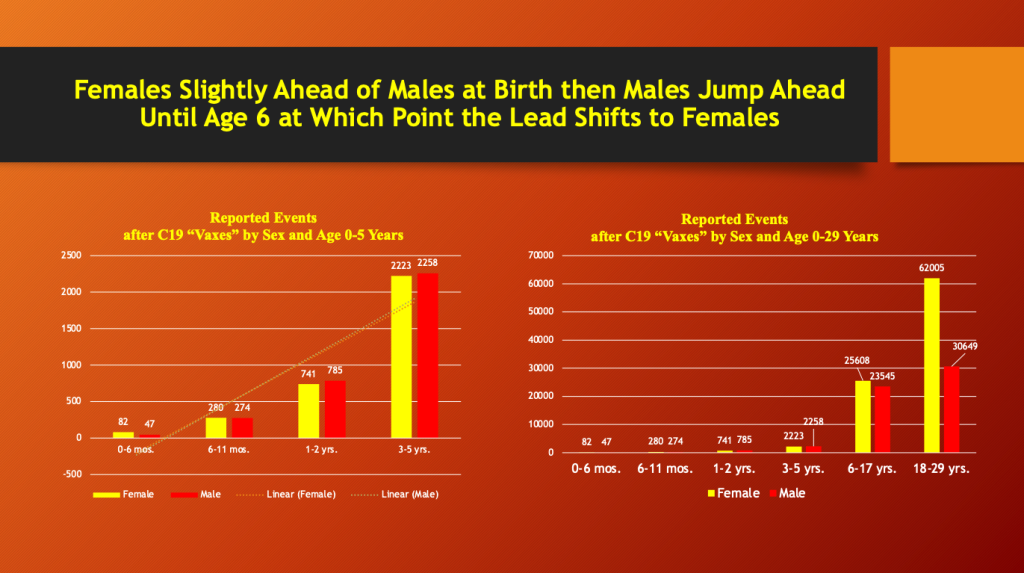
VAERS through 5/12/2023
Males and females have almost equal adverse event reporting from one to five years, at which age females take over and the lead never changes thereafter. There is a big increase in female predominance going from the six- to 17-year bracket to the 18- to 29-year bracket as the number of reports in females more than doubles. The age bracket from six to 17 years is too broad given the hormonal and physical changes occurring during this period. Age is reported in VAERS by age bracket although specific age by year data is recorded but is not directly retrievable using defined search terms.
A possible explanation for the female shift to a dominant position during adolescence and early adulthood may be related to female sexual development, specifically the onset of menarche. A CDC National Health Statistics Report from 2020 shows the age of onset of menarche follows the approximate time course of the emerging dominance of females in having reported adverse events from LNP/mRNA products. (https://www.cdc.gov/nchs/data/nhsr/nhsr146-508.pdf)
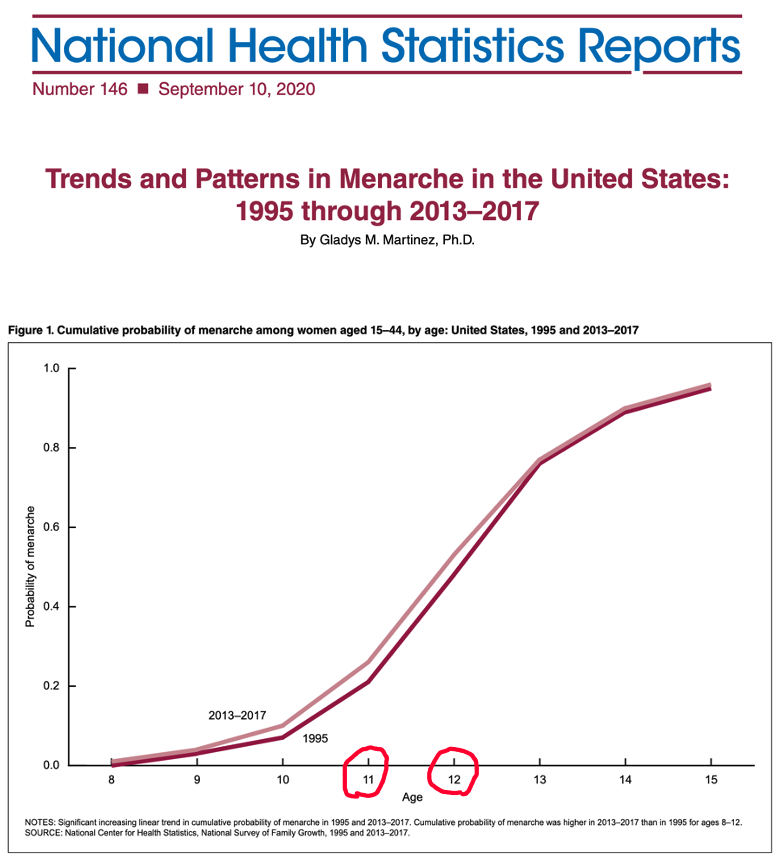
“The median age at menarche decreased from 1995 (12.1) to 2013–2017
(11.9). The cumulative probability of menarche at young ages was higher in
2013–2017 compared with 1995.”
Ed Clark from the War Room/DailyClout Pfizer Document Analysis Project was able to retrieve age-by-year data from VAERS for December 2020 through June 2023 with no filtering by vaccine type or manufacturer. Hormonal changes for females, below left, are ramping up during the pre-teen years as females begin to dominate adverse event reporting as shown below on the right.
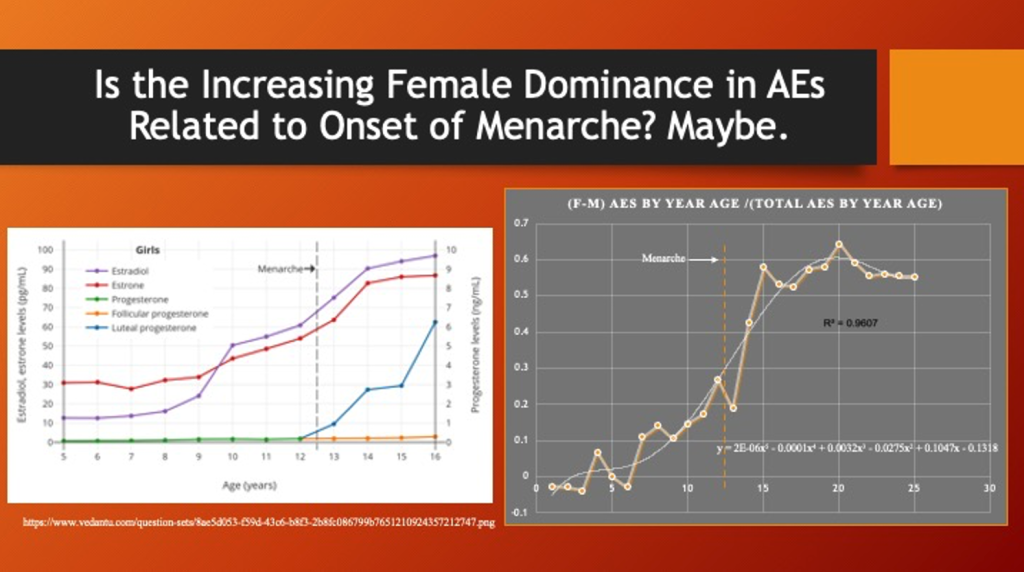
The polynomial function was used strictly to obtain the general trending of the data over the observed data range and should not be construed to predict outcomes beyond that range.
More granular data, age data by year rather than coarse age brackets, for the full data set in VAERS compared with quantitative hormonal changes might help further support or reject this hypothesis.
D. VAERS Events: Ages 40 and Above, Menopause
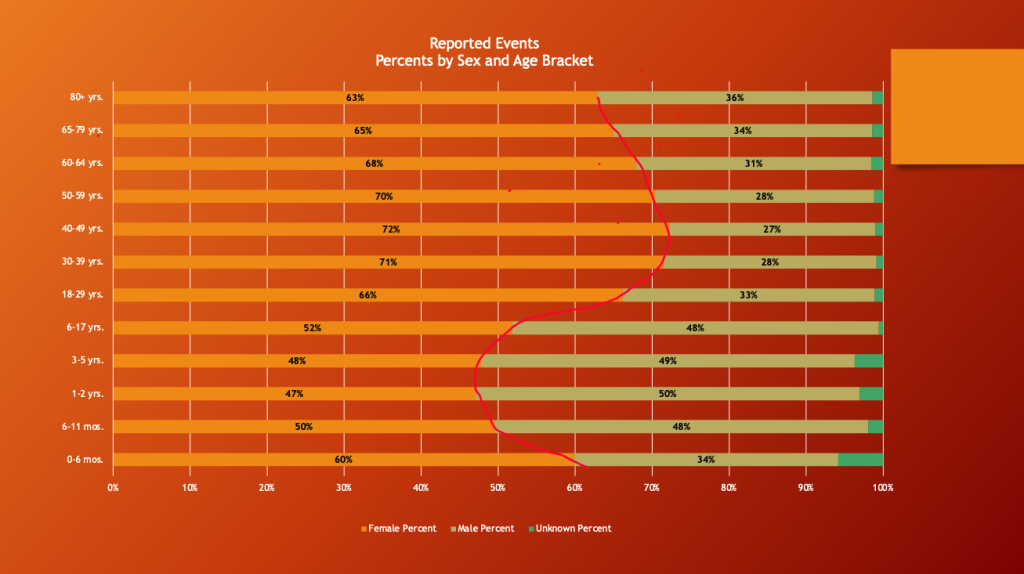
VAERS 5/12/2023
Women peak at 72% of adverse event reporting from ages 40 to 49 years, as menopause begins for many, and progressively drops down to 63% in the post-menopause age brackets.
“Spontaneous or natural menopause is recognized retrospectively after 12 months of amenorrhea. It occurs at an average age of 52 years, but the age of natural menopause can vary widely from 40 to 58 years.” (https://www.menopause.org/docs/default-source/2014/nams-recomm-for-clinical-care.pdf)
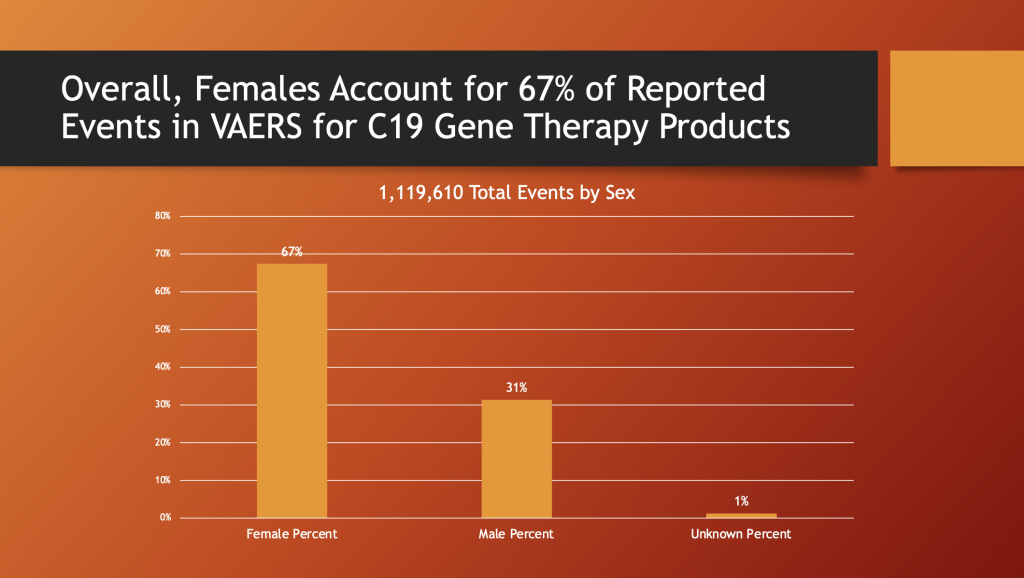
VAERS May 12, 2023
Overall, the women substantially dominate adverse event reporting in VAERS by more than a two-to-one margin.
E. VAERS Events: Disability after C19 Gene Therapy
Similar to adverse event reporting, women also have approximately a two-to-one lead in disability after adverse events after receiving C19 gene therapy products.
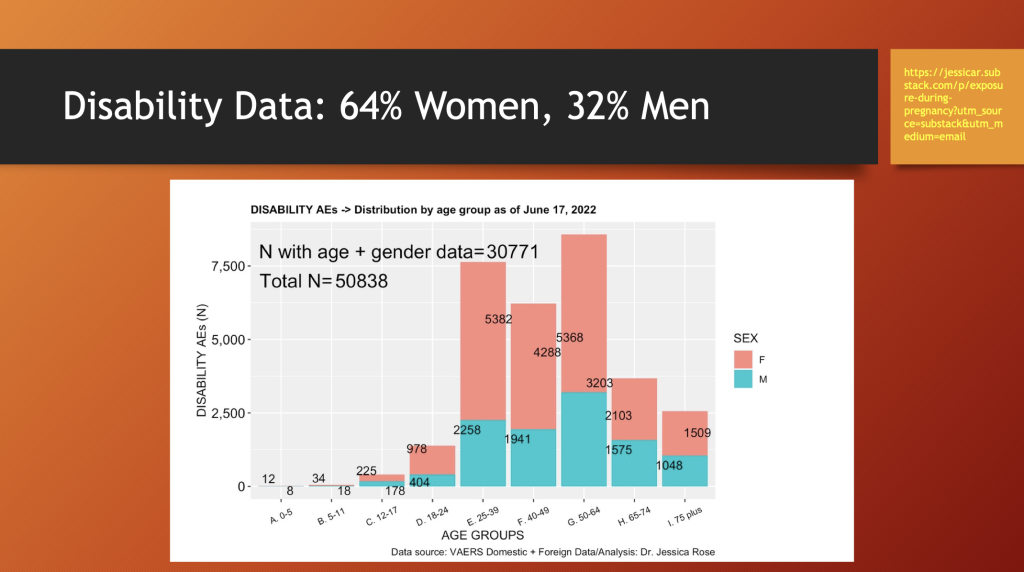
Jessica Rose prepared the chart above illustrating female preponderance in having disability from adverse events following C19 “vaccine” treatments across all age groups.
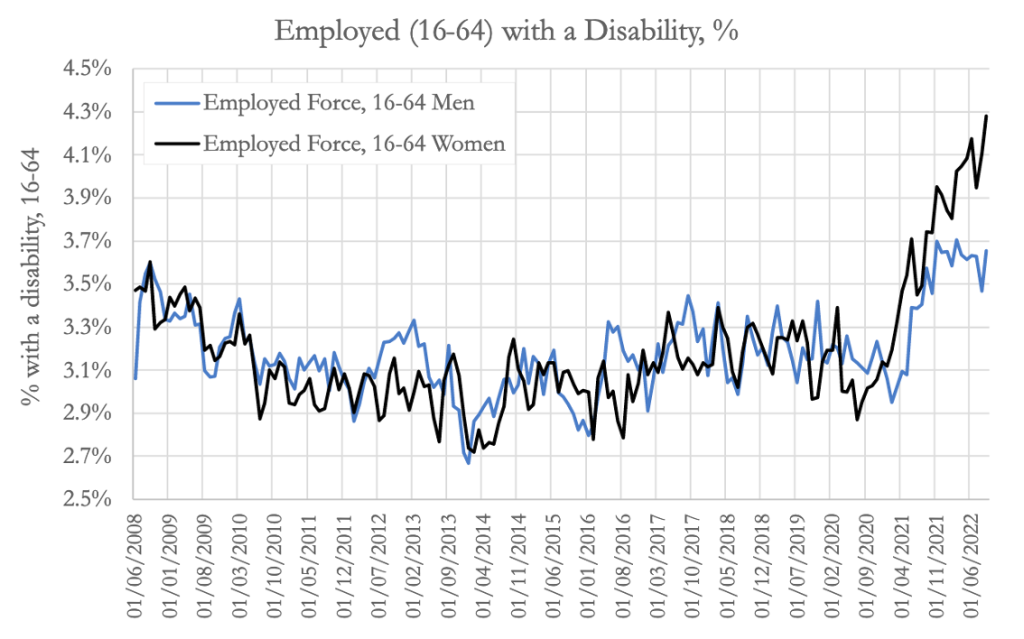
Ed Dowd’s group found similar pattern of women diverging sharply upward from men in disability event reporting in VAERS as the GTP program ramped up. (https://phinancetechnologies.com/HumanityProjects/US%20Disabilities%20-%20Part1.htm)
Below is a plot of Dr. Rose’s data showing men narrowing the gap in the 12- to 17-age bracket with respect to disability followed by female dominance of the statistic until the gap begins to narrow after age 50.
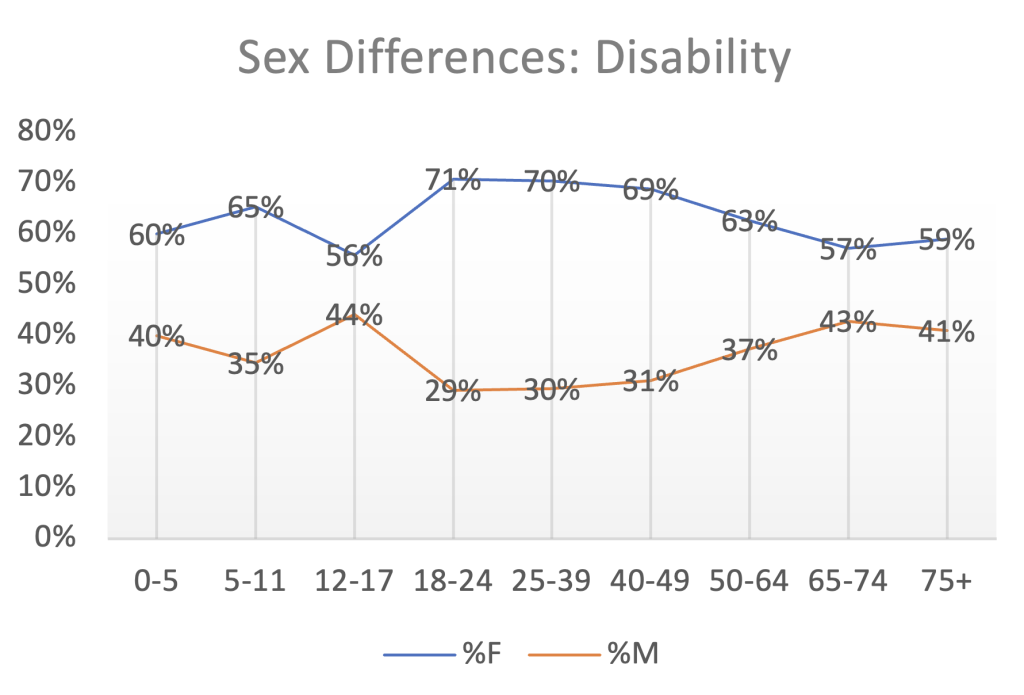
Females account for 64% of the disabled from adverse events in the entire data set of 30,532 disability event reports.
III. CoVax Disease and Youth: Conception Through Early Adulthood
This section will look at a number of significant medical problems following GTPs affecting youth beginning in utero.
A. In Utero, Miscarriage/Stillbirth
The recent release of Pfizer document, Appendix 2.2, reporting on the cumulative data collection through June 2022 and the interval of December 19, 2021, through June 18, 2022. (https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf) There were a total of 1,485,027 cases with 4,964,106 total adverse events.
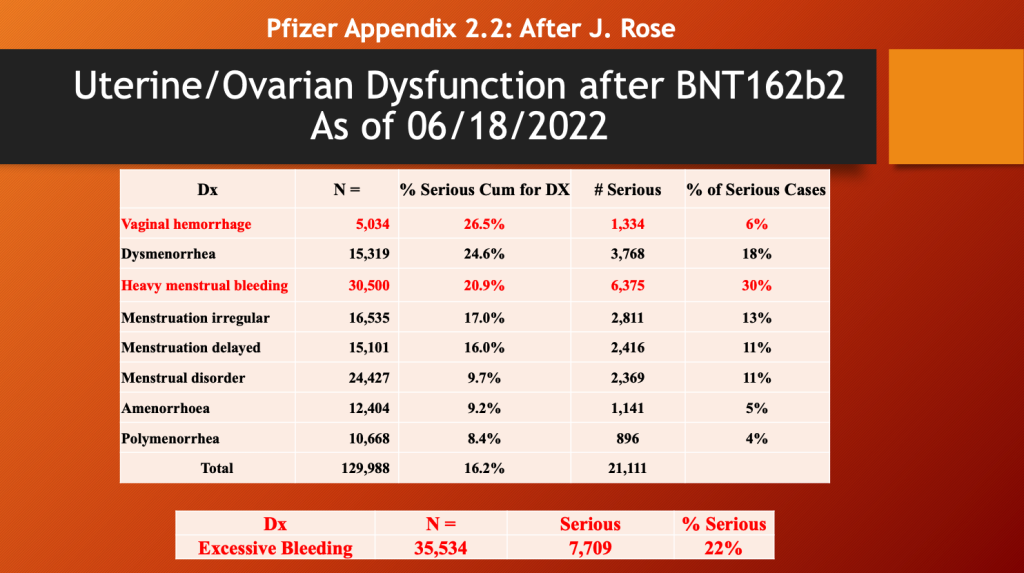
Dr. Rose has pulled out the following data from this document. (https://open.substack.com/pub/jessicar/p/pfizer-appendix-22-document-compared)
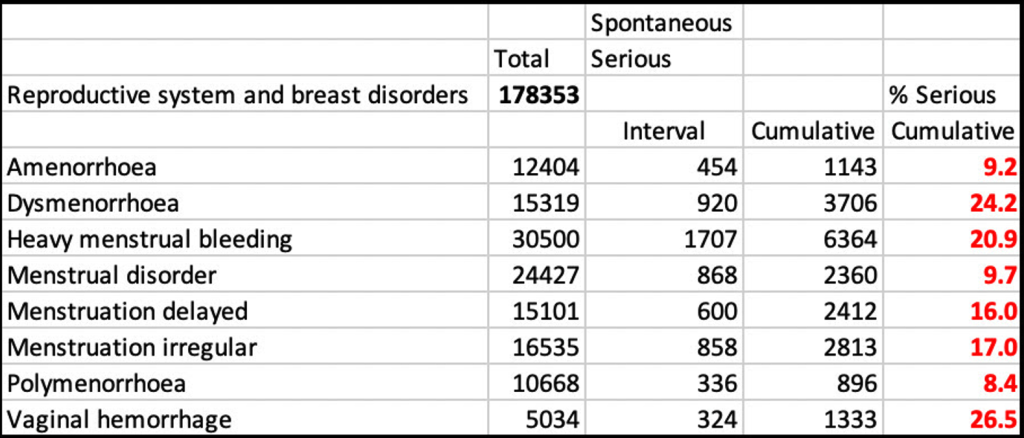
Female menstrual dysfunction was reported in 129,988 adverse events as of a year ago (06/18/2022 with 16% considered serious (below). There were 35,534 reports of excessive bleeding/hemorrhage with 22% of the events considered serious.
Interfering with normal ovarian/menstrual functions has consequences. Below is the data from OpenVAERS showing a spike in miscarriages and stillbirths as the LNP/mRNA dosing program ramped up.
The following table from OpenVAERS lists almost 5,000 miscarriages or stillbirths; 36,765 menstrual disorders; vaginal/uterine hemorrhage in 12,871; and over 1,000 fetal defects as of May 12, 2023.
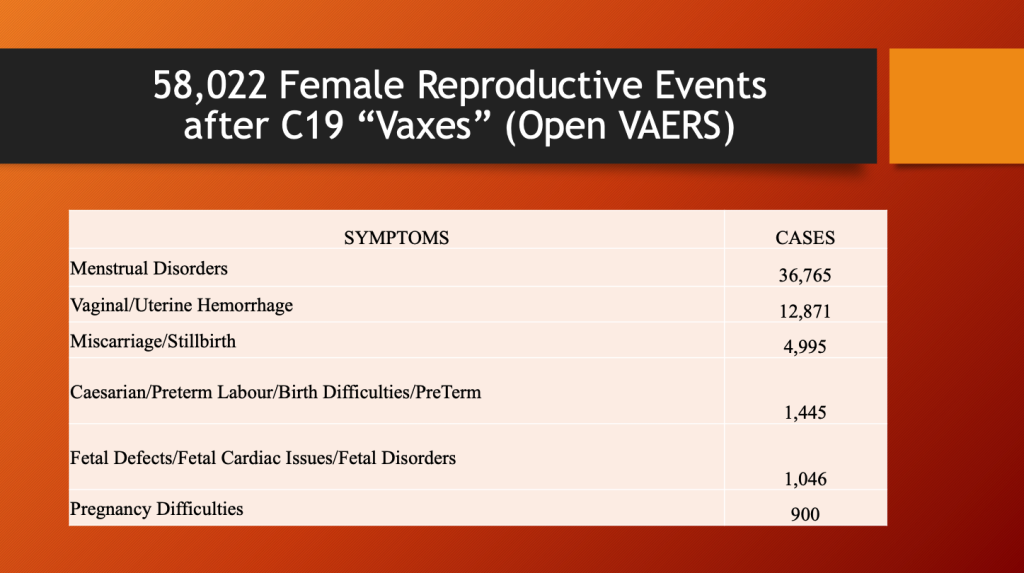
Applying the URF of 41 from Kirsch et al. gives the following estimates of the true numbers.
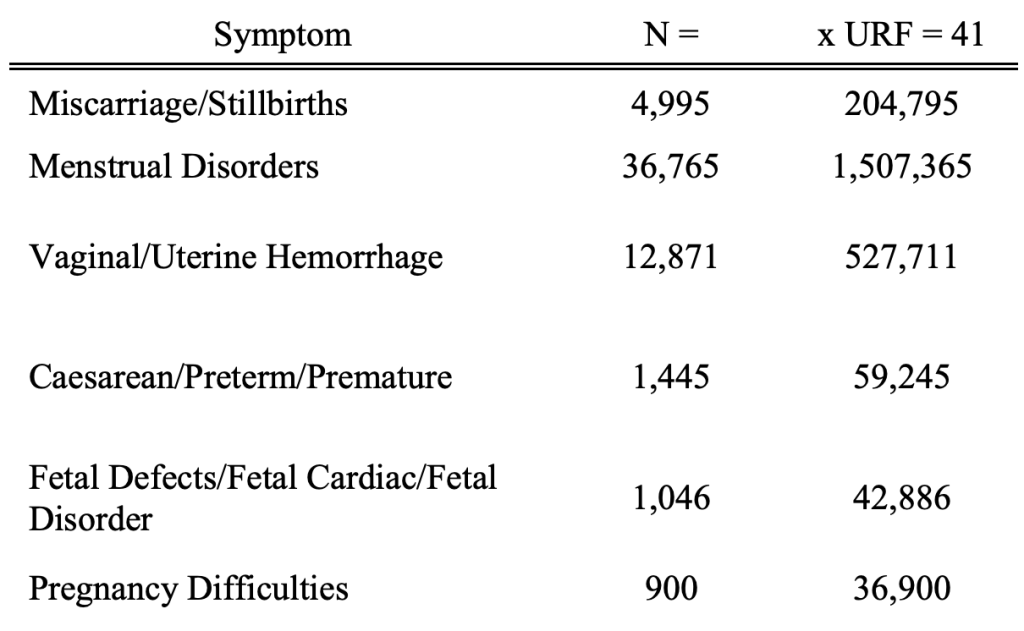
These are sobering numbers.
By comparison, it has been estimated that thalidomide caused 10,000 cases of birth defects in Europe from 1957 to 1961 before it was pulled from the market. Like spike-producing drugs, thalidomide was never tested in pregnant women and yet was aggressively marketed to them for morning sickness. (https://www.drugs.com/monograph/thalidomide.html)
Francis Kelsey of the FDA is credited with preventing use of thalidomide in the United States and, in doing so, ushered in the modern era at the FDA beginning in 1960 and ending in 2020 when the FDA/CDC shifted from protector to perpetrator. (https://www.fda.gov/about-fda/fda-history-exhibits/frances-oldham-kelsey-medical-reviewer-famous-averting-public-health-tragedy)
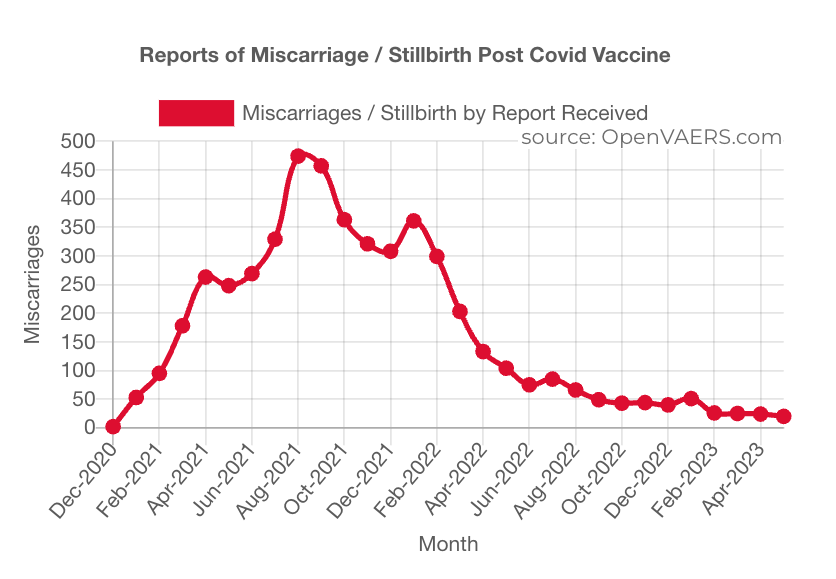
The miscarriage/stillbirth event reporting in VAERS shows a crescendo pattern as GTP population dosing ramped up in 2021 followed by a drop-off in 2022.
What is the physiological connection between perinatal adverse events for mother and child and the C19 gene therapy drugs? Dr. Arne Burkhardt, M.D., a recently deceased pathologist in Reutlingen, Germany, organized a 10-member team of pathologists, coroners, and scientists to study the histopathology of C19 vaccine organ and tissue damage in autopsy and biopsy specimens from about 130 subjects.
The Burkhardt Group’s seminal work establishing the pathological basis of CoVax Disease has been presented in Parts 1 and 2 of this series. (https://dailyclout.io/report-56-autopsies-reveal-the-medical-atrocities-of-genetic-therapies-being-used-against-a-respiratory-virus/, https://dailyclout.io/report-58-part-2-autopsies-reveal-medical-atrocities-of-genetic-therapies-being-used-against-a-respiratory-virus/)
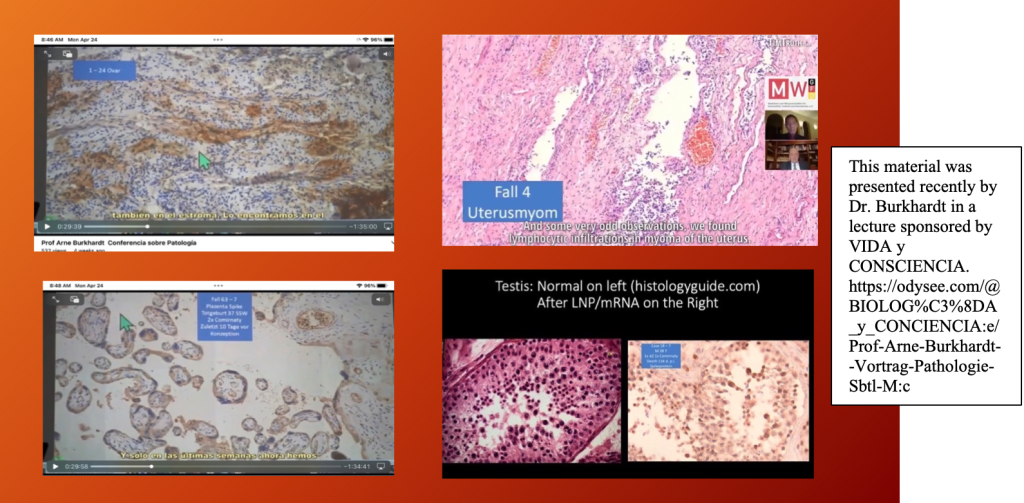
Dr. Burkhardt’s group identified the histopathology of harms associated with C19 gene therapy products in ovaries positive staining for spike protein Top Left, lymphocytic infiltration in the uterus (endometrium) Top Right, spike protein in placenta Bottom Left, and spermatozoa depletion in testes Bottom Right. The mechanisms of these harms were diverse and varied from vasculitis, protein deposition, inflammation, necrosis, and neoplasia.
Not surprisingly, GTPs have been linked to a decline in live births around the globe.
“Nine Months Post-COVID mRNA ‘Vaccine’ Rollout, Substantial Birth Rate Drops in 13 European Countries, England/Wales, Australia, and Taiwan.” (https://dailyclout.io/report-52-nine-months-post-covid-mrna-vaccine-rollout-substantial-birth-rate-drops/)
B. Neonatal/Breast Milk
Pfizer Confidential Document 5.3.6 reporting on the first 10 weeks of widespread use of BNT162b2 – up to February 28, 2021, in the U.S. and 12 weeks in the United Kingdom – identified a problem with nursing mothers who received BNT162b2 while breastfeeding:
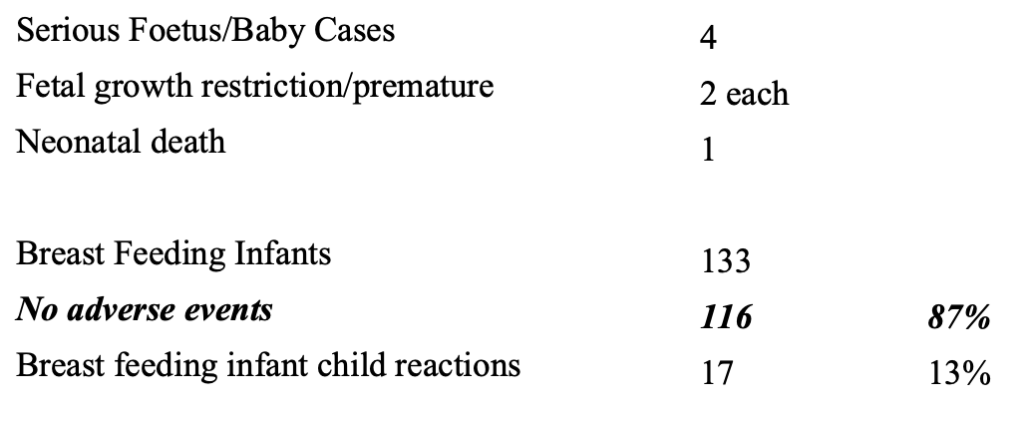
Keep in mind that follow-up for the “No Adverse Events” is not provided by the CDC, thus calling into question the accuracy of these numbers.
There were four serious fetus baby cases and one neonatal death. Thirteen percent of the 133 breastfeeding infants had 19 different reactions. (https://dailyclout.io/pfizer-evidence-so-far-coverups-heart-damage-and-more/)
Meanwhile, breastfeeding mothers experienced the following:

Given these reactions were observed early in the release of BNT162b2, it was not too surprising to see the study by Hanna et al. who reported detecting mRNA from C19 “vaccines” in breast milk.

(https://jamanetwork.com/journals/jamapediatrics/fullarticle/2796427)
“Of 11 lactating individuals enrolled, trace amounts of BNT162b2 and mRNA-1273 COVID-19 mRNA vaccines were detected in 7 samples from 5 different participants at various times up to 45 hours postvaccination (Table 2).”
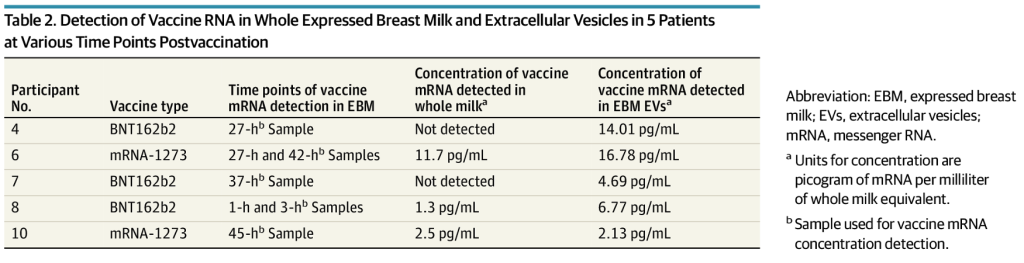
“Discussion
The sporadic presence and trace quantities of COVID-19 vaccine mRNA detected in EBM suggest that breastfeeding after COVID-19 mRNA vaccination is safe, particularly beyond 48 hours after vaccination [italics and bold added]. These data demonstrate for the first time to our knowledge the biodistribution of COVID-19 vaccine mRNA to mammary cells and the potential ability of tissue EVs to package the vaccine mRNA that can be transported to distant cells.” (Bold added.)
Remarkably the authors concluded that mRNA in breast milk was safe.
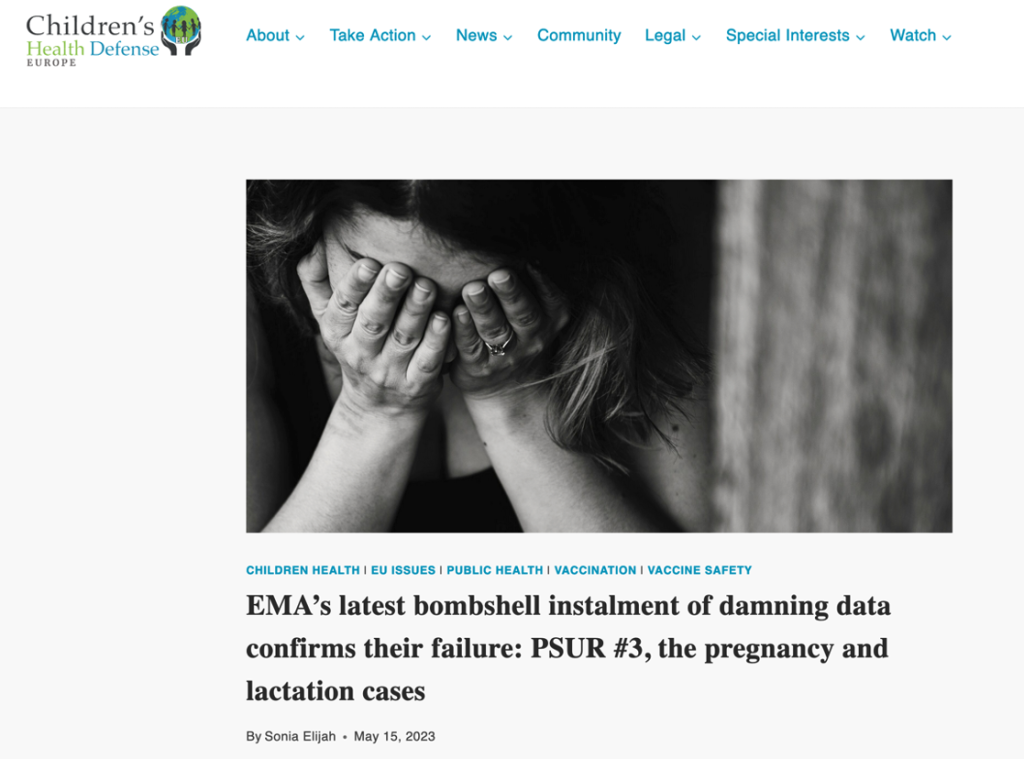
In May of this year a report was issued by Sonia Elijah in Children’s Health Defense Europe identifying in infants two cases of stroke after being exposed to mRNA-containing breast milk, three cases of severe neurologic disease, and four cases of respiratory Adverse Events of Special Interest (AESIs). (https://soniaelijah.substack.com/p/emas-latest-bombshell-instalment)
Meanwhile, a Freedom of Information Act (FOIA) request for records from the CDC turned up the following email in which John Su reported seeing “…a fair bit of ‘exposure by breast milk’- does that indicate an increase in reporting of this particular PT?” (https://jackanapes.substack.com/p/wake-up-and-smell-the-glitch-in-the)

The email goes on to note errors in administering the C19 drug products to children aged five- to 11-years-old. This subject will be addressed more fully in Section IIIC, on administrative errors, to follow.
John Su goes on to note “vaccine failure” was on the increase, as was known from Pfizer Confidential Document 5.3.6, February 28, 2021.
VAERS contains documentation of 38 cases of symptoms reported after breastfeeding infants’ exposure to mRNA from their mother’s milk:
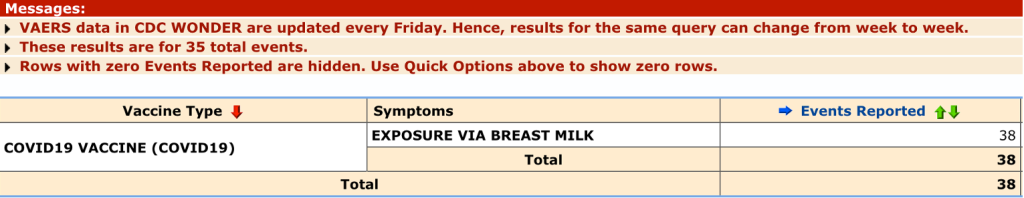
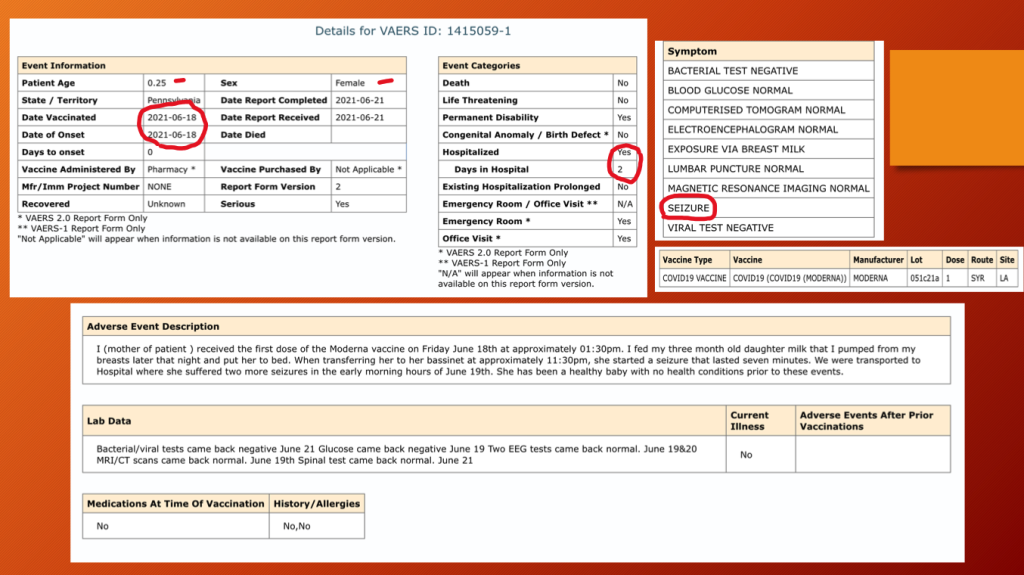
VAERS ID 1415059 (below) documents a three-month-old female who received mRNA1273 in her mother’s breast milk and had a seizure lasting seven minutes the same day as her mother’s inoculation. She was hospitalized for two days.
The report was completed three days after the seizure with no outcome information other than the child was considered to have permanent disability. The CDC does collect outcome information but does not share it.
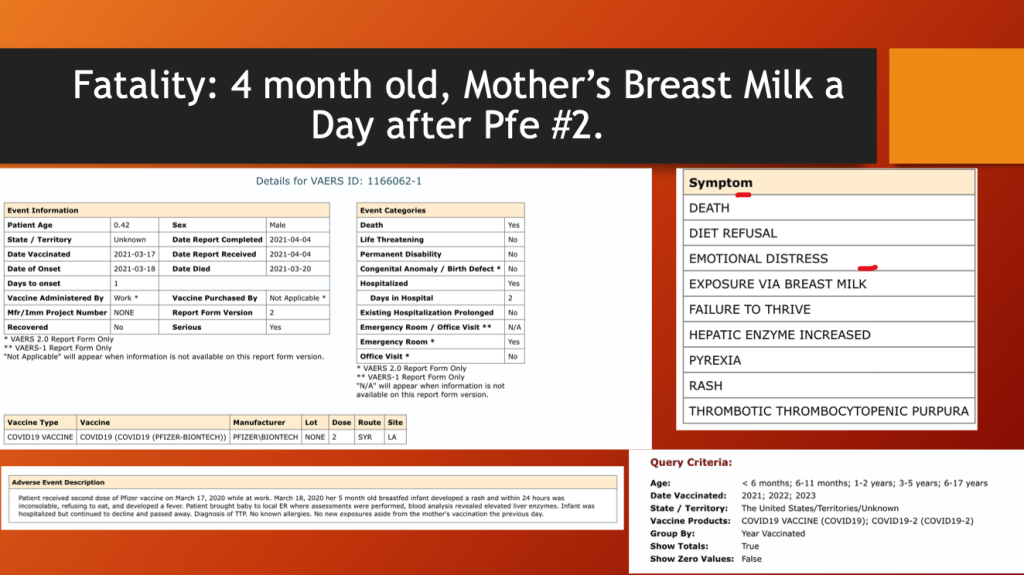
VAERS ID 1166062 (below) was a four-month-old male who died three days after his mother’s second dose of BNT162b2. The cause of death was failure to thrive, fever, and a hematologic condition known as thrombotic thrombocytopenic purpura.
These are a sample of similar cases in VAERS. Dr. Makis has published a recent article on LNP/mRNA-related neonatal fatalities. (https://open.substack.com/pub/makismd/p/mrna-and-pregnancy-infants-who-died)
C. Administrative Issues Have Consequences: Ages Zero to 17 Years
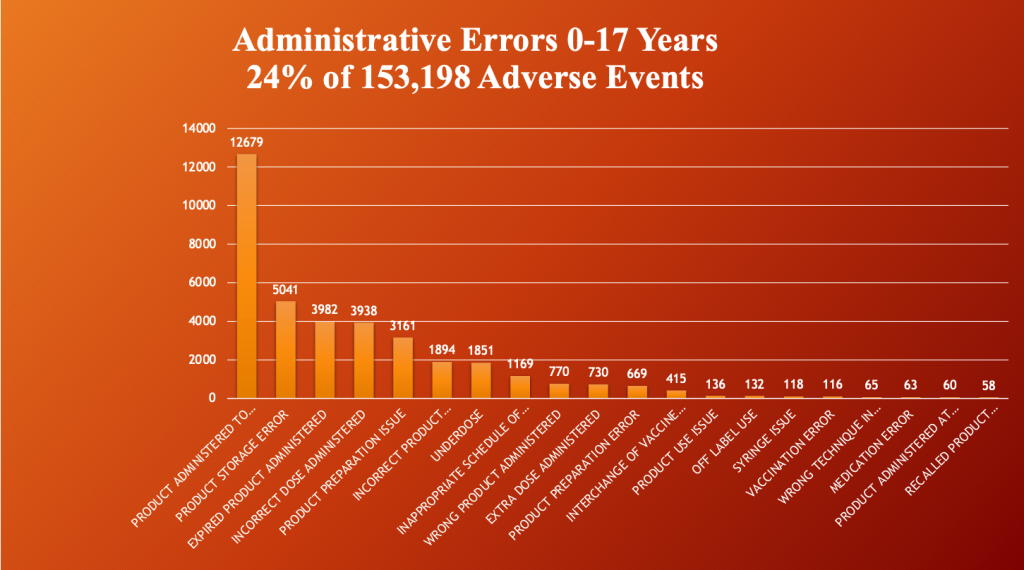
VAERS through 5/12/2023 C19 Gene Therapy Drugs U.S. and Territories.
Administrative errors occurred in 24% (37,235/153,198) of all reported events in children ages zero- to 17-years-old. (VAERS) The most common by far is administration of the product to children and adolescents too young to receive the drug.
Was this a byproduct of the $1 billion campaign to scare and cajole parents into having their children injected to keep them safe?
Follow-up on these administrative errors, like the one below, has not been posted if it exists.
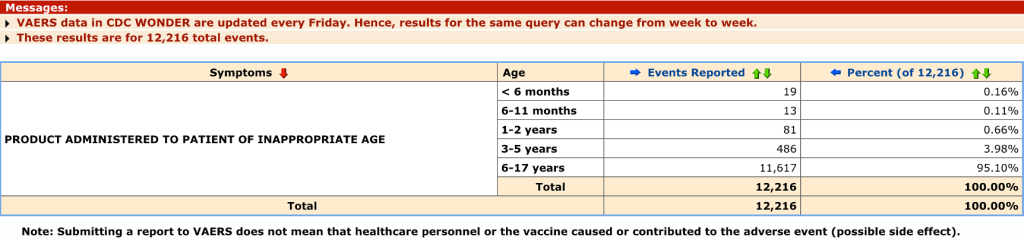
Some of these errors have had disastrous complications.
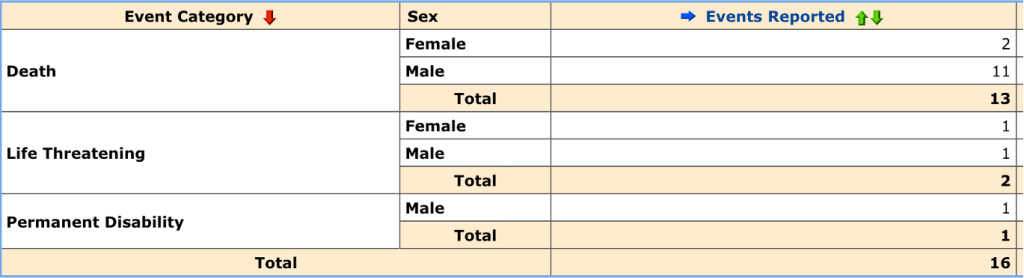
This search result gives more detail on the “No Adverse Effects,” Death, Life Threatening, and Permanent Disability.
VAERS ID 2457513: This 15-year-old girl had an injection that was coded “Product Administered to Patient of Inappropriate Age”. After her second dose of mRNA-1273 (Moderna), she had a cardiac arrest and died. Why was a 15-year-old receiving an injection to “prevent” C19 classified as a “PATIENT”?
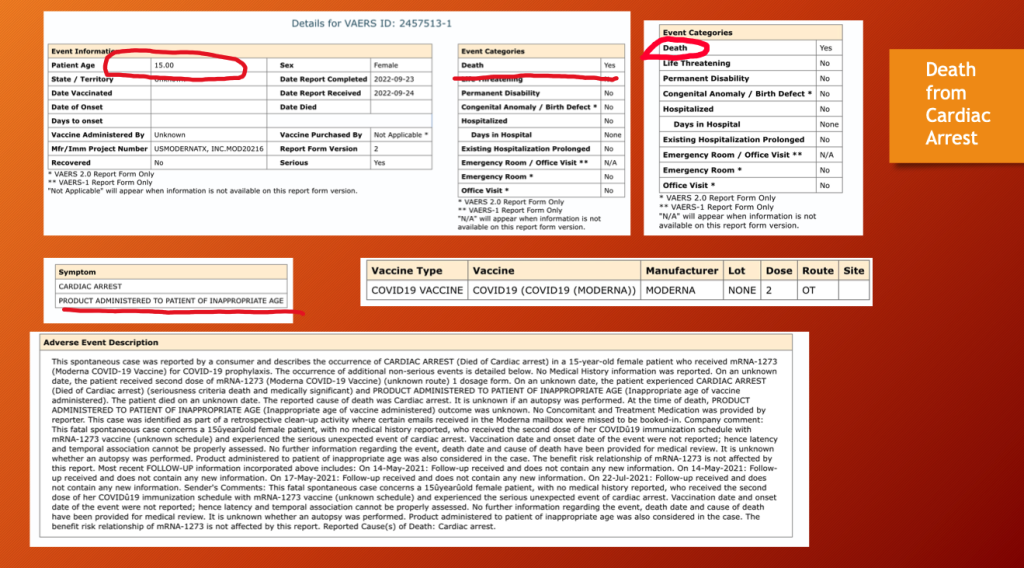
“The benefit risk relationship of m/RNA-1273 is not affected by this report.” Why would anyone say something like that after a 15-year-old, otherwise healthly girl died after receiving a failed “vaccine”?
VAERS ID 1772015, “Inappropriate Schedule of Product Administration”, also concerns a 15-year-old — this time a boy.
Four days following dose two of Pfizer’s BNT162b2, the teen developed multifocal hemorrhagic lesions in his brain: cerebrum, brainstem, and cerebellum.
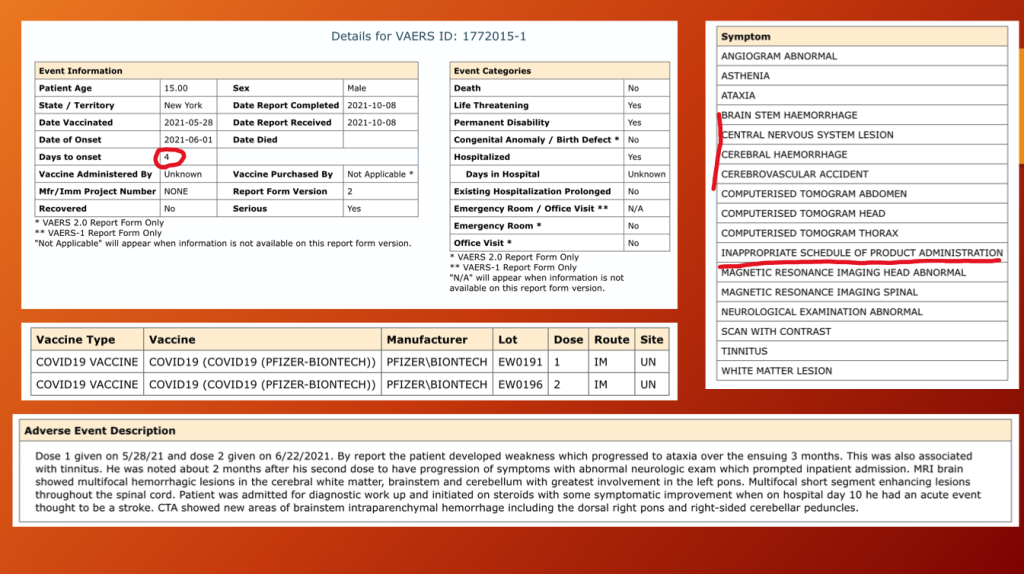
He was considered permanently disabled at age 15 years.
The topic of administrative errors and their consequences is worthy of a separate report given that there were over 37,000 of them. Applying the URF of 41, that works out to 1,526,635 medication errors of various types.
D. Cardiac Arrest
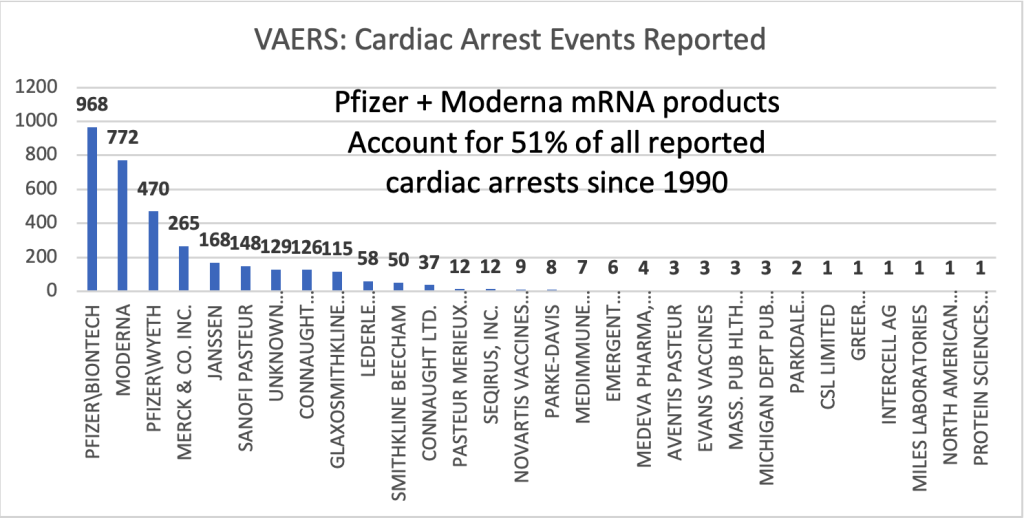
VAERS through 5/12/2023
Across all ages and since 1990, the mRNA products account for more than half of all vaccine-related cardiac arrests in VAERS.
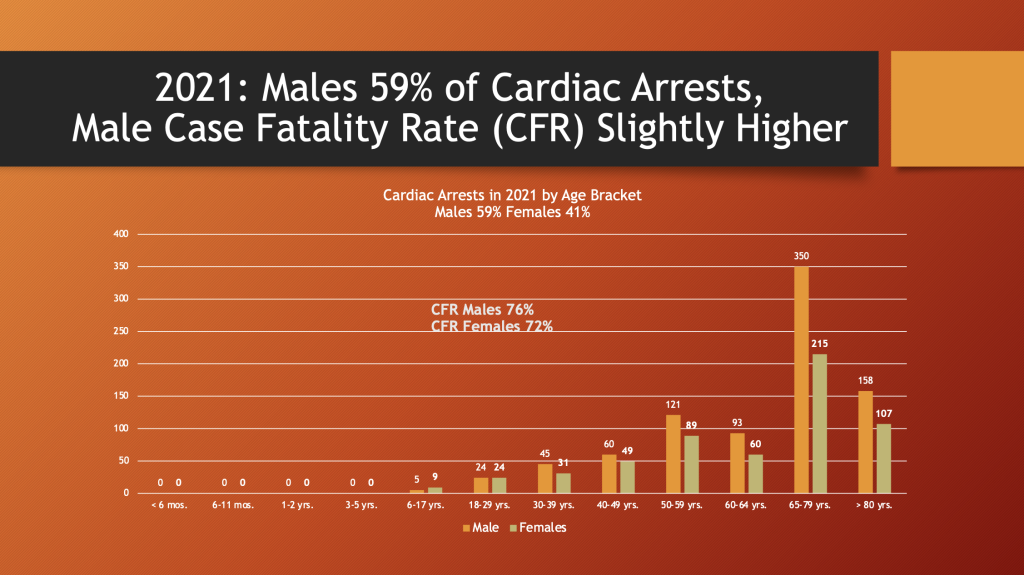
Males had 59% of the cardiac arrest events reported to VAERS in 2021, the first year of the GTPs.
The fatality rate was 72% for females and 76% for males. The age bracket from 65 to 79 years of age had the greatest number of reports. There were 62 event reports for < 30 years-of-age. With an URF of 41 that results in 2,501 cardiac arrests in this age bracket.
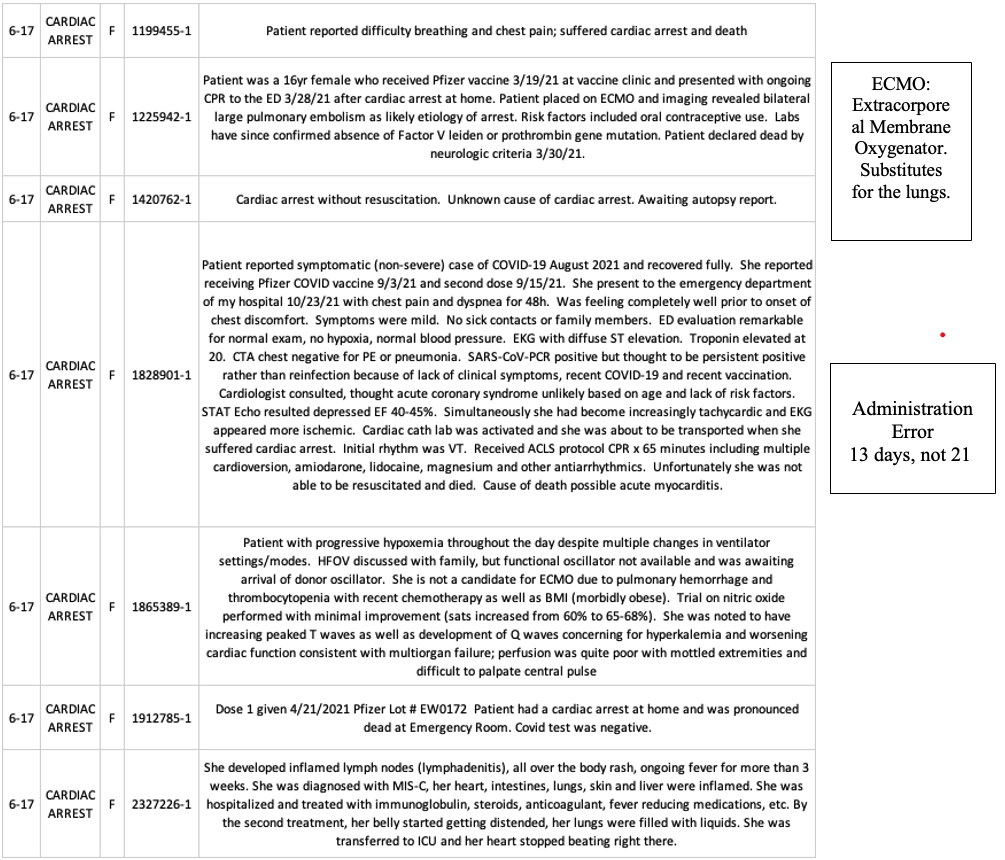
The following cases from VAERS are typical cardiac arrest cases, except these are children (ages six to 17), not septuagenarians.
E. Central Nervous System and Neurological Disease: Co-VAN Cluster
Samim et al. performed an extensive literature review of neurologic diseases associated with the C19 gene therapy drugs and organized them under the heading of Co-VAN (COVID-19 vaccine-associated neurological diseases) as illustrated below.
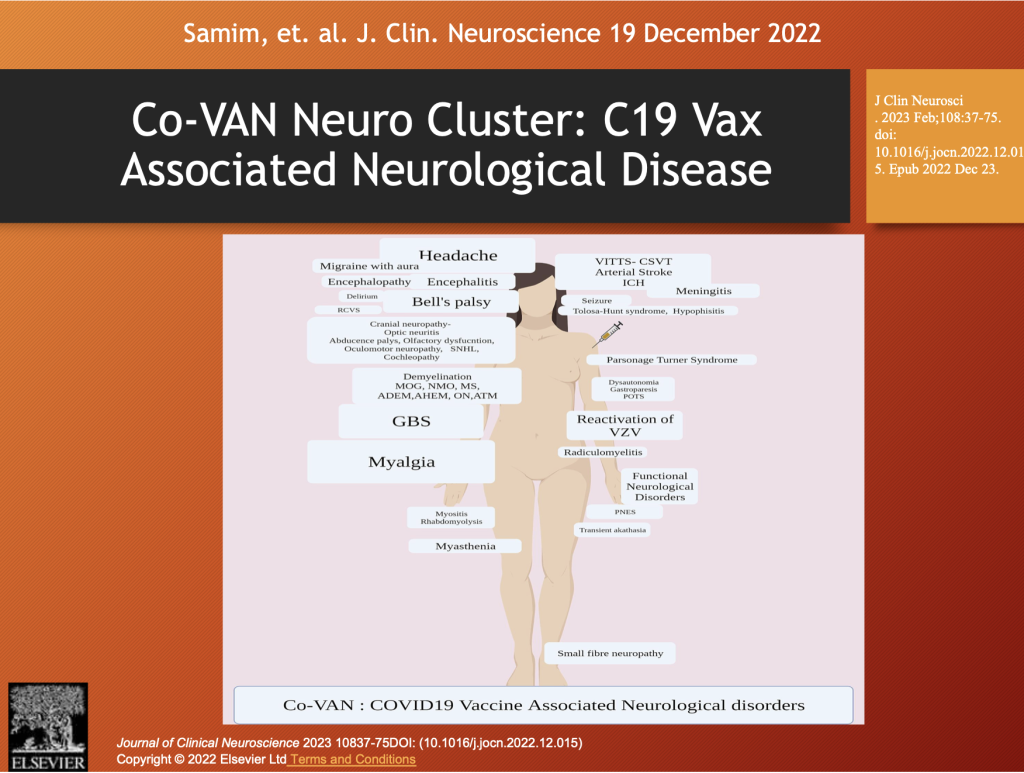
The Co-VAN Disease group fits well in a taxonomy of CoVax Disease based on organ systems and pathological processes associated with spike-generating drugs. This topic will be further developed in Part 5 of this series.
There is wide variation in the manifestations of neurological disease following spike-generating drugs from peripheral neuropathy to demyelinating disorders to stroke, seizures, encephalitis, protein deposition disease, and cranial neuropathies, such as Bell’s Palsy or Ramsay Hunt Syndrome (cranial nerve VII).
Pfizer Confidential Document 5.3.6 summarized adverse events and Adverse Events of Special Interest (AESIs) from December 2020 through February 2021. The following histogram summarizes the neurological diseases identified from a pool of over 40,000 GTP subjects reporting complications after receiving BNT162b2.
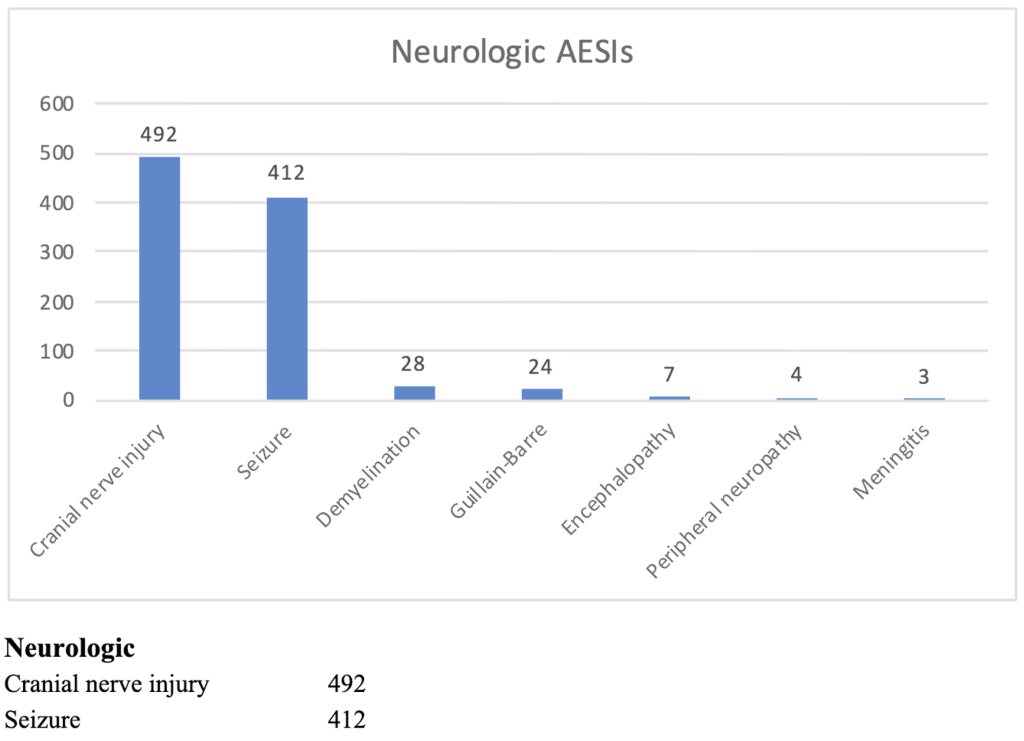
In spite of this significant “signal,” C19 gene therapy products were mandated by governments throughout the world. Not surprisingly, neurologic complications appeared in children as OpenVAERS summarizes below.

https://openvaers.com/covid-data/child-summaries
Overall, there were 5,220 neurological events ranging from 4,549 with migraine to 49 with cerebral hemorrhage/aneurysm in ages six months to 17 years. The estimates with an URF of 41 are given below.
These are significant medical problems that often leave permanent impairment and disability.
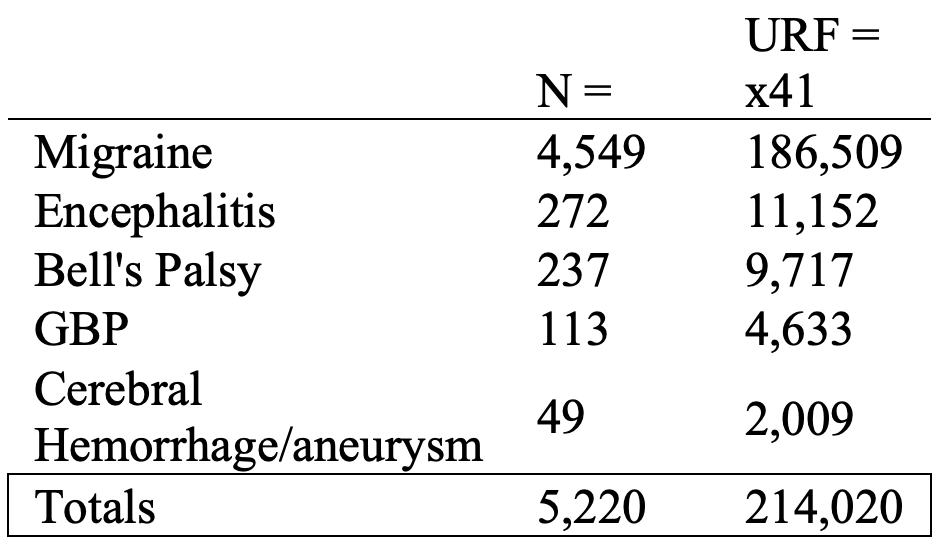
i. Acute Disseminated Encephalomyelitis (ADEM)
ADEM is a rare autoimmune demyelinating central nervous system disease, typically associated with patients younger than 15 years of age. Encephalitis is an inflammatory condition of the brain tissue known as myelin (the tissue coating nerve fibers and maintaining normal function of the nerves). Dr. Burkhardt’s collection contains histopathological evidence of inflammation of both brain and the membranes around it.

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10043599/
Brock et al. present the following case of a 10-year-old, previously healthy female who presented with progressive lower extremity weakness, paresthesia, and urinary retention.
“CASE REPORT
A 10-year-old female with no past medical history presented to Golisano Children’s Hospital, Fort Myers, Florida, United States on December 21, 2021 with 7 days of progressive lower extremity weakness, paresthesia, and urinary retention. No recent symptoms of infection were reported. Neurological examination showed mild lower extremity hyperreflexia, right lower extremity weakness with inability to ambulate, a mild pronator drift, and a right visual field defect. The patient received her second dose of mRNA-based COVID-19 vaccine 16 days prior to the onset of symptoms.
Upon admission, a comprehensive laboratory assay including CBC, CMP, ESR, and CRP, was negative. A respiratory viral panel which included SARS-COV2 PCR testing was negative. Contrast-enhanced MRI of the brain demonstrated multiple prominent T2/FLAIR hyperintense subcortical and deep white matter lesions with incomplete rim-enhancement, compatible with active demyelination, and avid peripheral diffusion restriction (Figure 1)” Emphasis added.
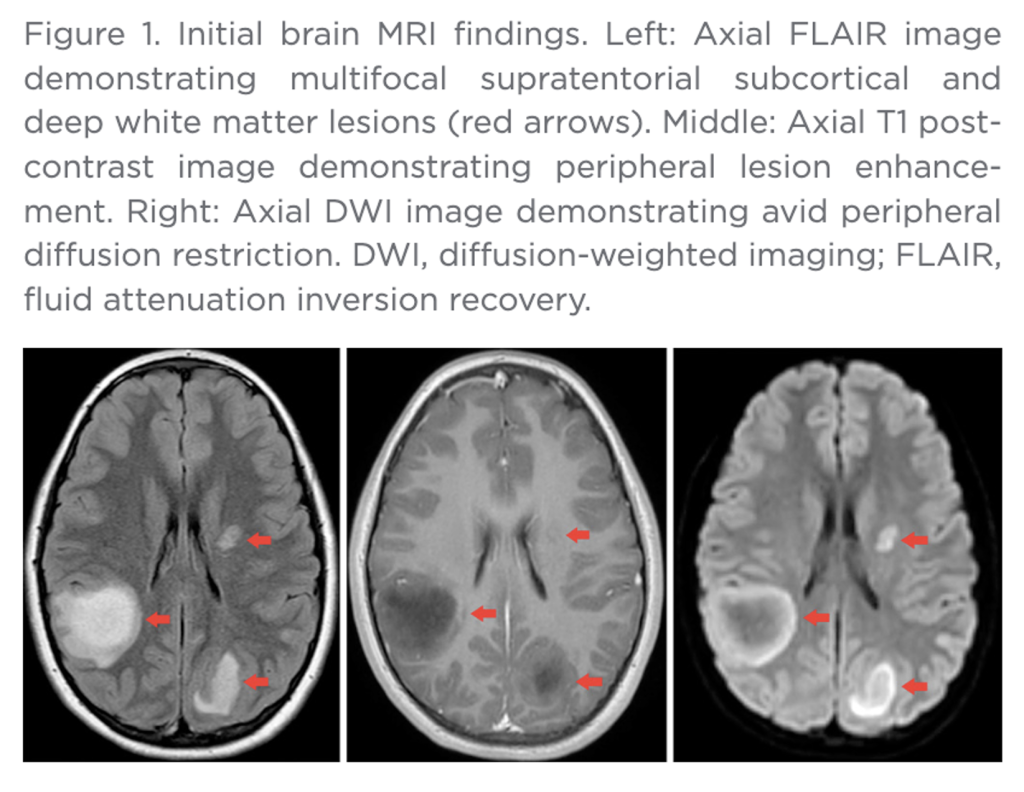
“Contrast-enhanced MRI of the cervical, thoracic, and lumbar spine demonstrated a long-segment, non-expansile, partially enhancing intramedullary lesion within the thoracic spinal cord, also most likely compatible with active demyelinating process (Figure 2). There were no findings suggestive of Guillain-Barré syndrome. Secondary differential considerations included transverse myelitis, CNS lymphoma, and atypical multiple sclerosis.
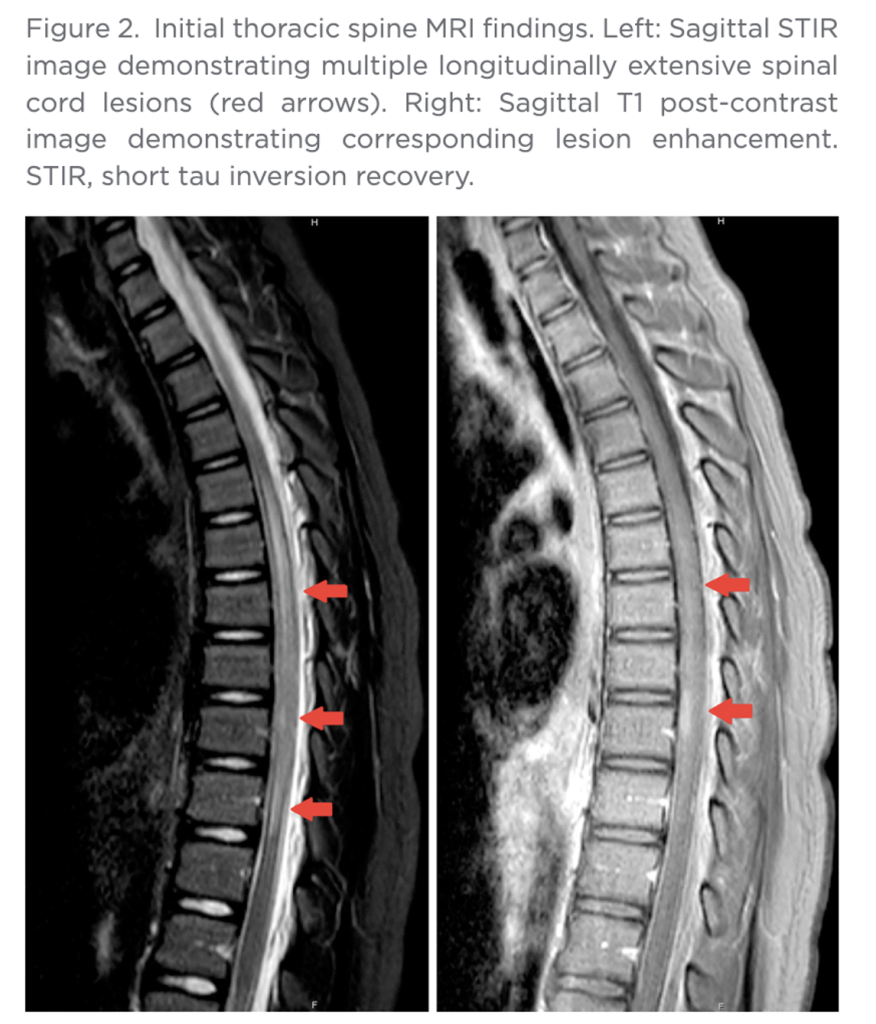
Three months after initial evaluation, the patient returned for outpatient neurologic follow-up. She reported mild fatigue. Examination showed a mild, persistent gait instability and hip muscle weakness. All other symptoms were resolved. A few days later, final histopathology showed white matter neurons with an extensive macrophage-rich inflammatory infiltrate with small lymphocytic component forming perivascular aggregates (Figure 3).”
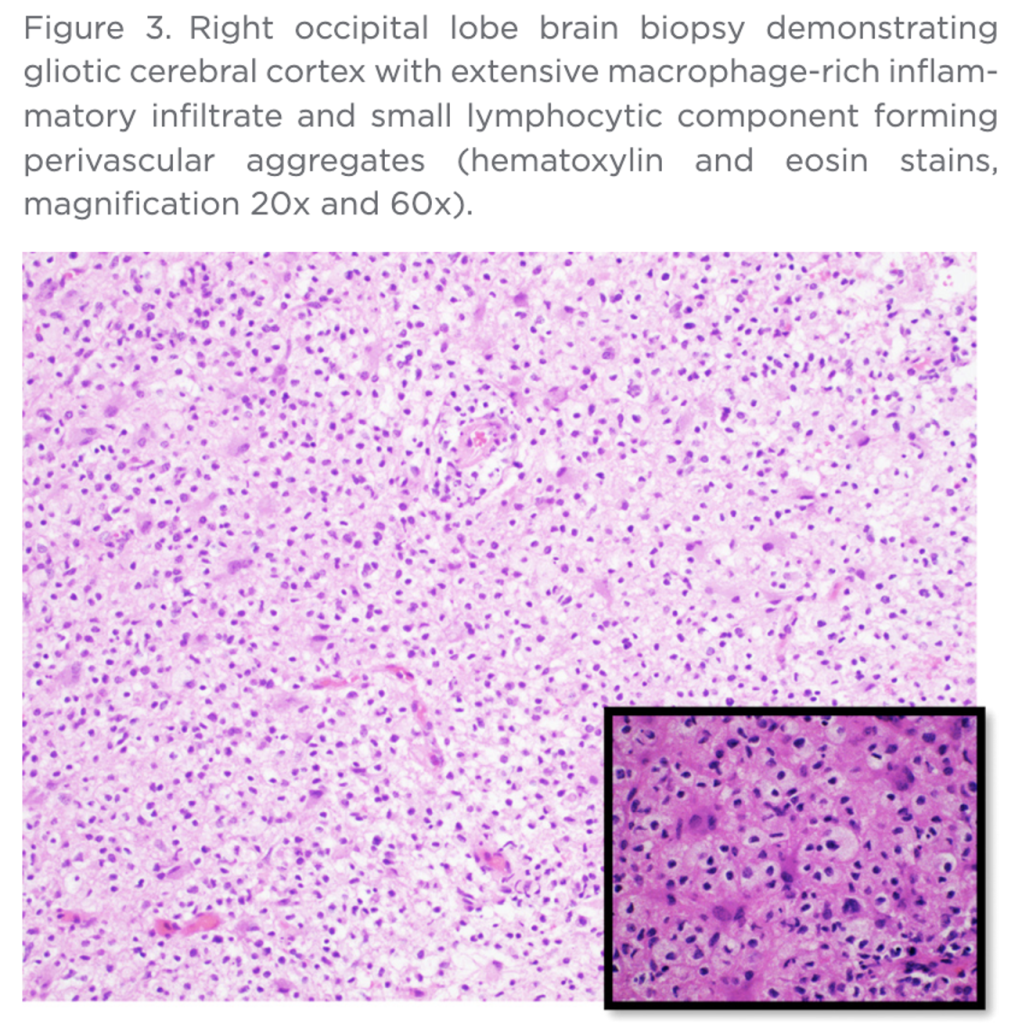
“Luxol/H&E stain showed severe loss of myelin, with macrophages demonstrating phagocytosed myelin debris. Immunohistochemistry showed depletion, but relative preservation of axons, with scattered perivascular accumulations of CD3+ T-cells and CD4/CD8 co-expressing T-cells” (Bold added.)
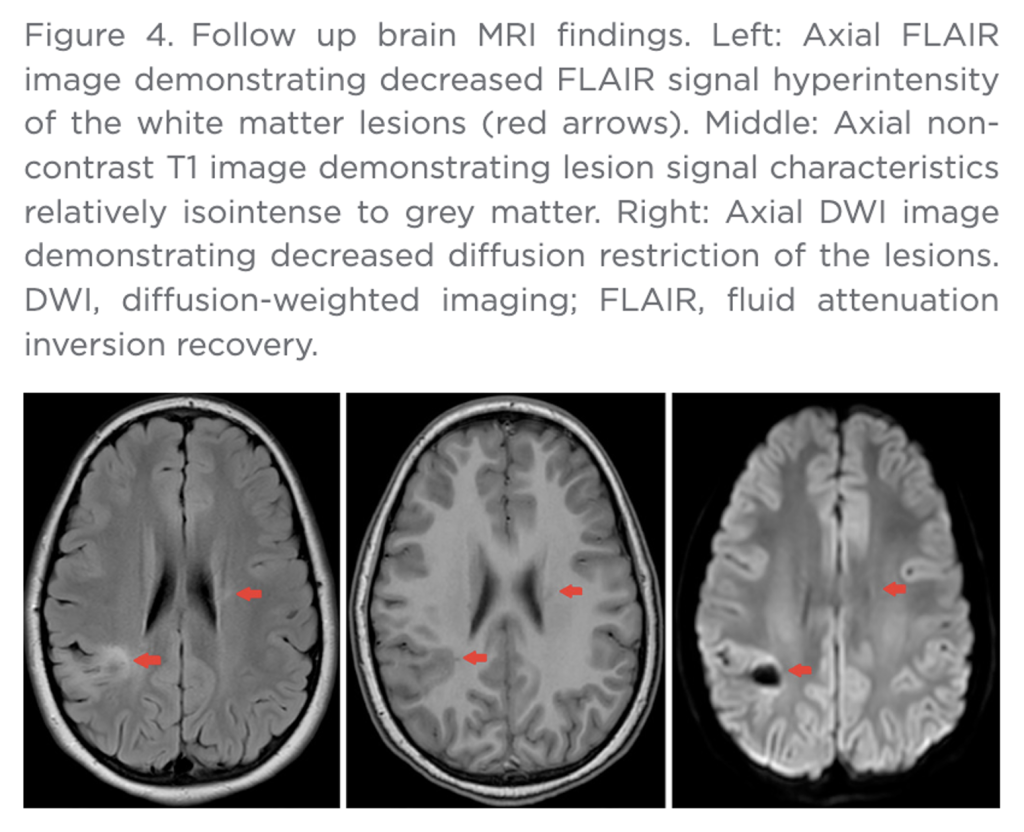
Widespread neurologic damage is well-documented in this case. Residual impairment and disability are probable.
VAERS ID 1948381 concerns an 11-year-old male with onset of ADEM three weeks after injection of flu vaccine along with BNT162b2 on November 17, 2021. MRI evaluation revealed abnormalities throughout the cerebral cortex with extension into adjacent tissues. The child was admitted to the hospital for five days during which he received high-dose steroids.
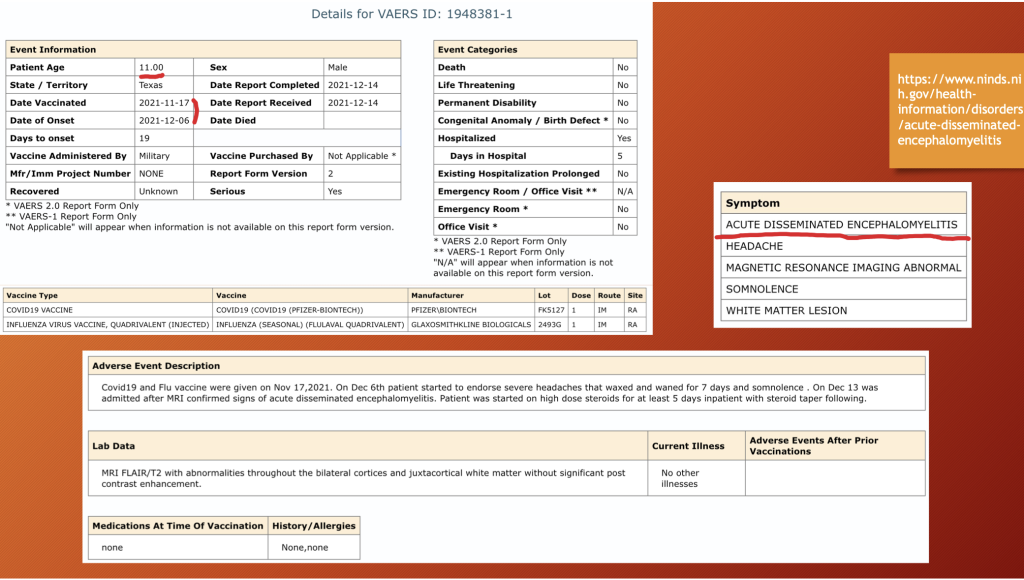
The report was filled out on December 14, 2021, while the child was still in the acute phase of his illness which began on December 6, 2021, only three days following hospital discharge; so, no outcome determination was possible even though the report indicated no death or disability. Residual impairment examination after one year is required.
ii. Aneurysm/Cerebral Hemorrhage
The pathomechanism of spike-producing drug-associated aneurysm was well described by Dr. Burkhardt’s Group in Parts 1 and 2 of this series. Briefly, vascular endothelium (inner lining) is attacked by either spike and related proteins or by activated leukocytes that disrupt the inner lining of an artery which leads to a rupture of the vessel wall, dissection of blood by arterial pressure through the muscular layer with dilatation of the vessel wall or rupture, or both.
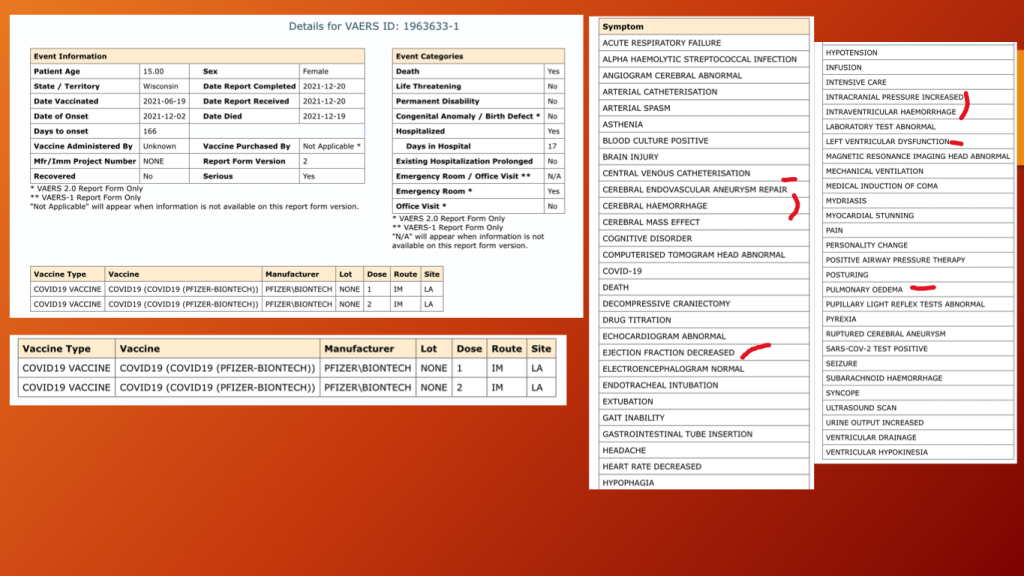
VAERS ID 1963633: This 15-year-old girl received her second dose of BNT162b2 on June 19, 2021, and passed away on December 17, 2021, after a very complicated 17 days in the hospital.

The consequences of an aneurysm can be dire not only for the injured but the loved ones who suffer while they helplessly watch as their child dies.
iii. Stroke
The father’s words in the image below tell the story pretty well.

Childhood stroke has been reported to occur in 1 to 13 per 100,000 children. (Pediatric Stroke: A Review, Tsze and Valente, Emerg Med Int. 2011; 2011: 734506. Published online 2011 Dec 27. doi: 10.1155/2011/734506 PMCID: PMC3255104 PMID: 22254140, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255104/.) Unfortunately, denominators to properly calculate prevalence rates are lacking with GTPs.
Pfizer Confidential Document 5.3.6 reports a seven-year-old child who received BNT162b2 long before pediatric dosing was developed and long before the emergency use authorization (EUA) was extended to children. This is another example of the sloppy administration of the “vaccine” program.
Dr. Makis and Professor Miller, independently of one another, maintain archives of CoVax Disease cases culled from the media (links below).
and
https://markcrispinmiller.substack.com
Edward Dowd’s book, Dowd, Ed. “Cause Unknown”: The Epidemic of Sudden Deaths in 2021 & 2022. Skyhorse Publishing, 2023 is a similar archive of sudden deaths along with statistical data.
F. Autoimmunity and Immunologic Effects
Autoimmunity was featured in Part 4C of this series. (https://dailyclout.io/report-70-part-4c-clinical-evidence-supporting-the-hypothesis-that-autoimmunity-is-one-of-the-principal-pathological-mechanisms-of-harm-from-covid-19-gene-therapy-drugs/)
Briefly, autoimmunity is a process in which one’s immune system attacks “self” thus producing an illness through the process of inflammation, often with system-wide effects although more localized varieties exist such as thyroiditis and diabetes type I.
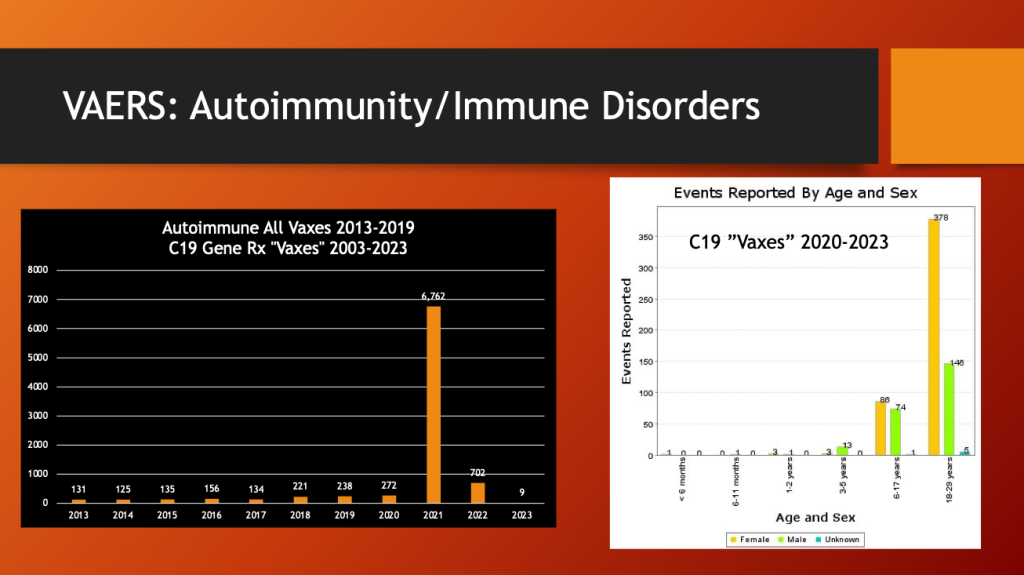
Eighty-five percent of autoimmune diseases following vaccination in the past 10 years have followed the public introduction of C19 gene therapy drugs. Females dominate the 18- to 29-year-old bracket.
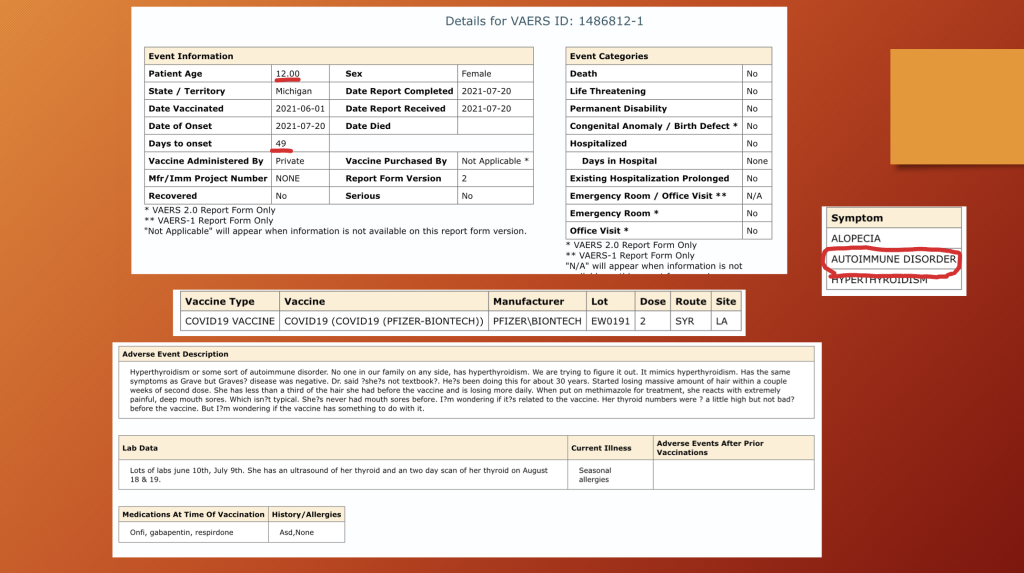
VAERS ID 1486812: This 12-year-old girl developed autoimmune thyroiditis 49 days after dose two of BNT162b2. CoVax illnesses often resemble known illnesses, but, as in this case with “massive” hair loss, something is unique in many of them.
G. Multisystem Inflammatory Syndrome in Children (MIS-C)
MIS-C is an inflammatory process that involves multiple organs and physiological systems simultaneously or in sequence, sometimes mild at first but proceeding rapidly at times to critical illness. MIS-C was discussed in Part 4C of this series. (https://dailyclout.io/report-70-part-4c-clinical-evidence-supporting-the-hypothesis-that-autoimmunity-is-one-of-the-principal-pathological-mechanisms-of-harm-from-covid-19-gene-therapy-drugs/)
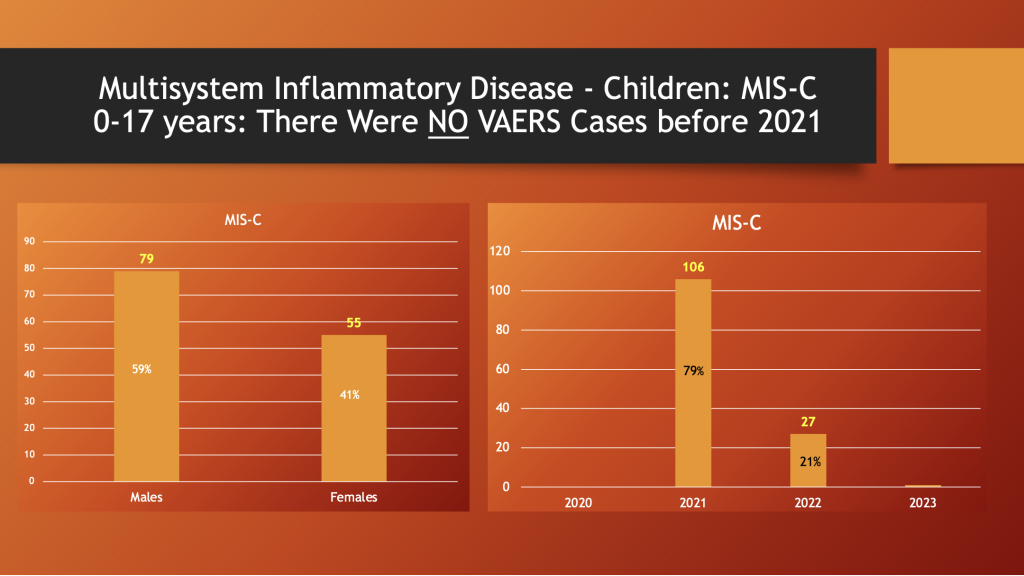
VAERS through 5/12/2023
Males are afflicted in 59% of reported cases (left histogram). There were no cases reports from 2020, but 106 were reported in 2021 and then, in 2022, the reports dropped to 27 as the spike drug use declined (right histogram).
As noted, prior to rollout of the C19 “vaccines,” there were no MIS-C cases in VAERS. As the public’s willingness to participate in the inoculation with C19 “vaccines” waned, so did the reports of MIS-C as shown in the right histogram above.
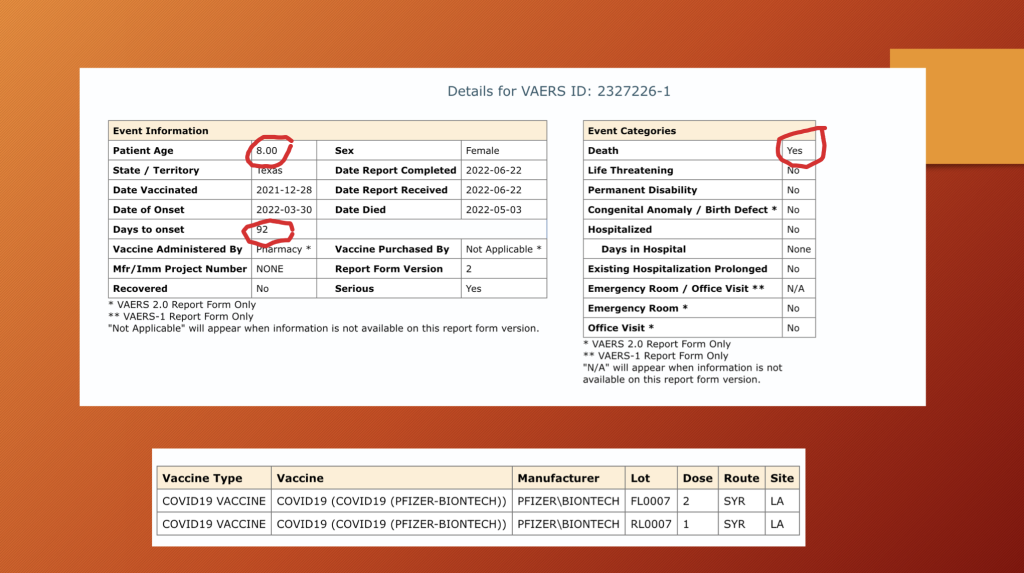
VAERS ID 2327226: This eight-year-old girl was not so fortunate as others and passed away four months following her second dose of BNT162b2. She was febrile for three weeks before being admitted to the hospital for multiple organ system failure.
Her organ involvement included lymph nodes, skin, heart, intestines, lungs, and liver.
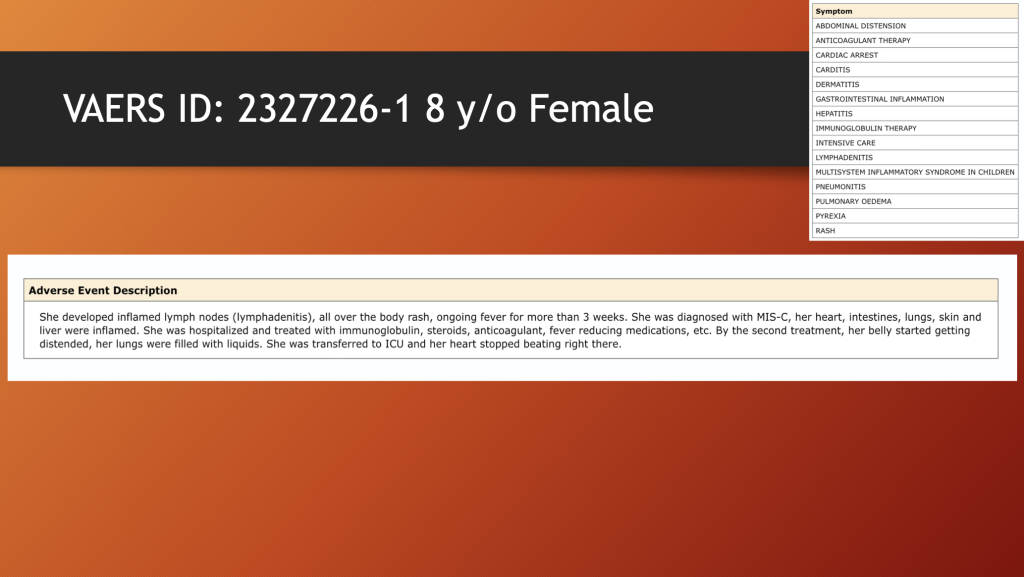
The final two sentences need emphasis: “By the second treatment, her belly started getting distended, her lungs filled with liquids. She was transferred to ICU and her heart stopped beating right there.”
Six cases from VAERS are summarized below. All six of these youngsters survived. There has been no assessment and reporting of impairment and disability in survivors.
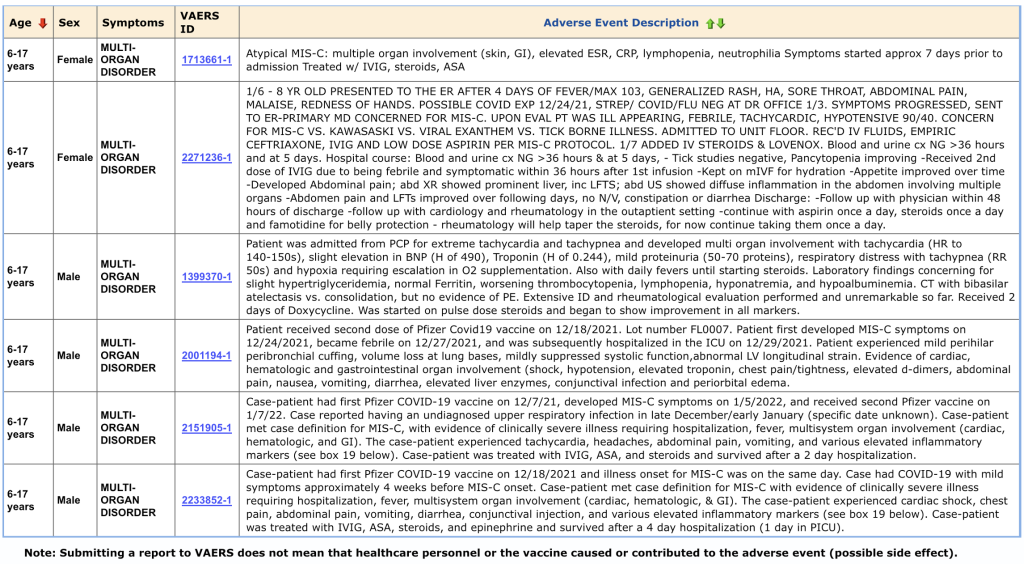
This category of CoVax Disease, MIS-C, is another example of complex and aggressive illness following GTPs.
H. Myocarditis/Pericarditis
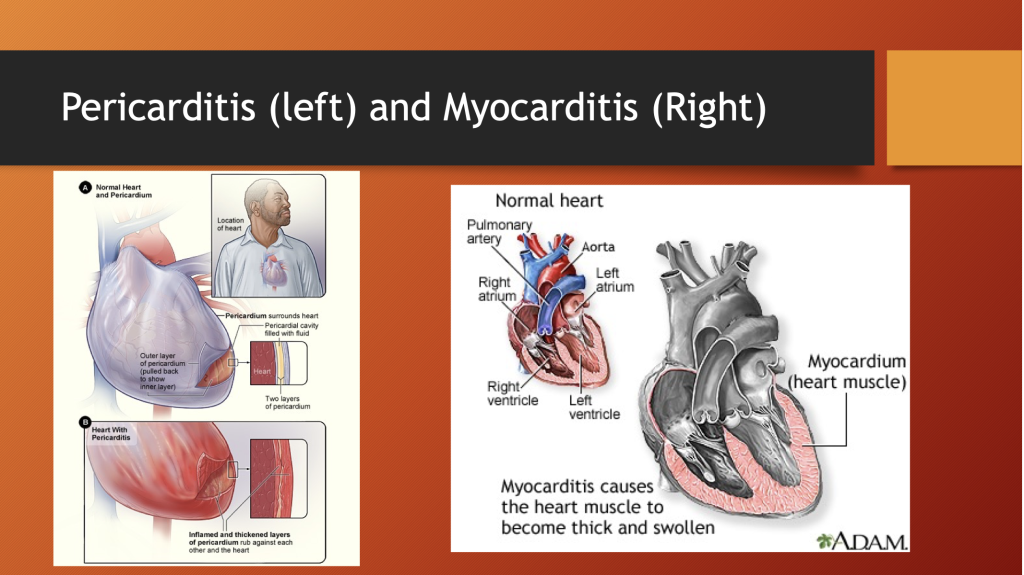
The pericardium is a fibrous tissue layer surrounding the heart. Inflammation of this sac-like structure can compromise cardiac function.
Myocarditis is inflammation of the heart muscle itself. Actual tissue destruction occurs to variable degrees; and, like any muscle, once the muscle cell dies, the muscle is replaced by rigid scar tissue.
Inflammation of the heart not only damages the muscle but can interfere with transmission of electrical signals to activate the heart muscle causing an irregular rhythm that can be fatal.
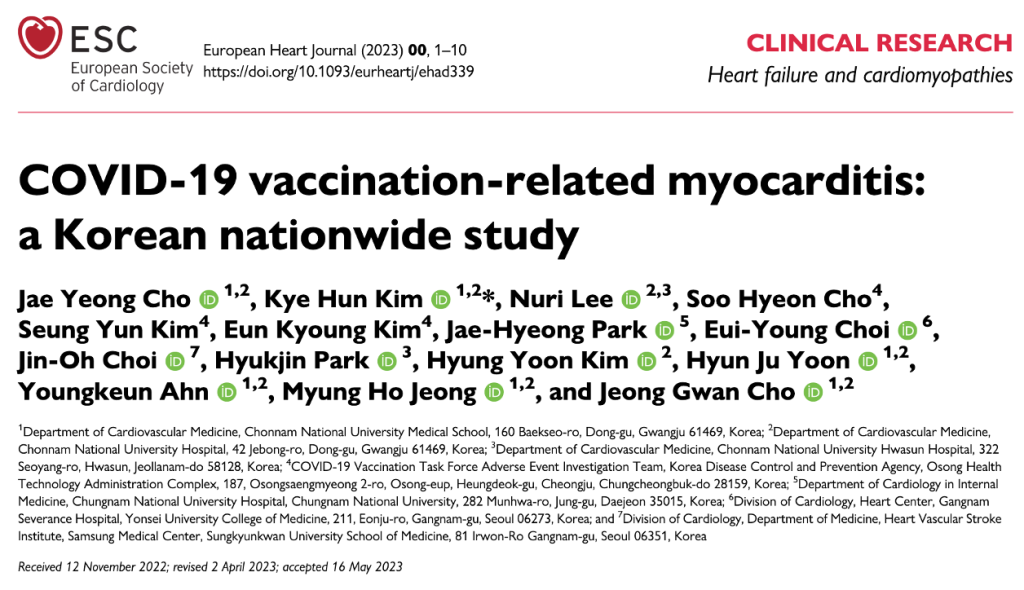
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehad339/7188747
Cho et al. reviewed the Korean national database reports of 1,533 cases of myo/pericarditis in the Korean vaccinated population of 44,276,704 persons, out of 51,349,116 (comprising the total Korean population) following review by the government-organized Expert Adjudication Committee on COVID-19 Vaccination Pericarditis/Myocarditis. After screening, the committee confirmed 480 cases of vaccine-associated heart disease.
Males accounted for 62% of cases with a median age of 30 years and a range of 20 to 45 years. ICU admission was required in almost 18%, and 0.2% underwent heart transplantation. Death occurred in 4.4%. The authors offer incidence estimates of 1.08 cases of vaccine-related cases of myo/pericarditis cases per 100,000 vaccinated persons. National dosing data is not a suitable denominator for prevalence calculation.
Cho et al. discussed under-reporting as a limitation of the study, “…thorough measurement of cardiac troponin levels and endomyocardial biopsy could minimize underreporting in the present study”. Detailed prevalence studies using cardiac MRI (cMRI) with late gadolinium enhancement (LGE), echocardiography, and other diagnostic studies are necessary.
Recently, Barmada et al. evaluated 23 young patients with myo/pericarditis. The bulk of the article concerns analysis of the immunological aspects of these cases, but they also presented one of the larger series of myopericarditis cases with follow up cMRI data.

https://www.science.org/doi/full/10.1126/sciimmunol.adh3455
Males comprised 87%, and the average age was 16.9 years, (range 13 to 21 years). Onset of symptoms ranged from a few days to over a week after the second dose of BNT162b2. Outcome data is contained in Supplement 1 found online but was lacking necessary clinical information including, but not limited to, age, follow-up time period, and functional recovery including athletics.
Six patients were excluded, but the authors admitted these cases might be worth studying as much as the others. The reasons for exclusion were a positive polymerase chain reaction (PCR) test for SC2 or greater than seven days between injection and onset of symptoms even though about a third of myocarditis cases in VAERS occur after seven days.
The six excluded patients were not differentiated from the six patients with incomplete cMRI data.
Blood studies were positive for myocardial injury and inflammation; elevated troponin, CRP, BNP, and NLR. (https://www.ahajournals.org/doi/full/10.1161/circulationaha.111.023697, https://www.mayoclinic.org/tests-procedures/c-reactive-protein-test/about/pac-20385228, https://my.clevelandclinic.org/health/diagnostics/22629-b-type-natriuretic-peptide, https://pubmed.ncbi.nlm.nih.gov/26878164/.)
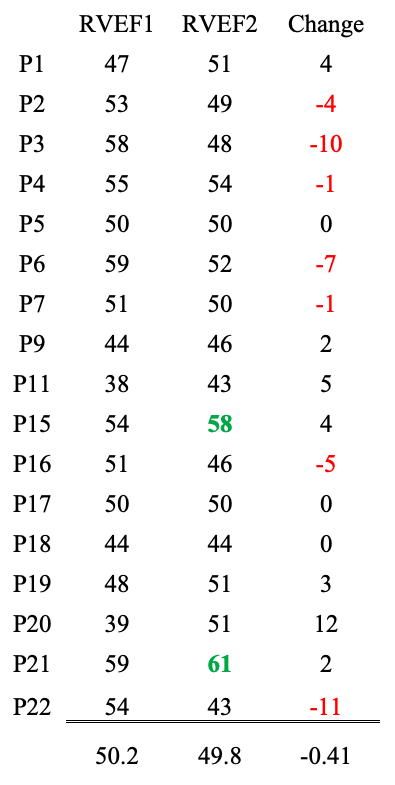
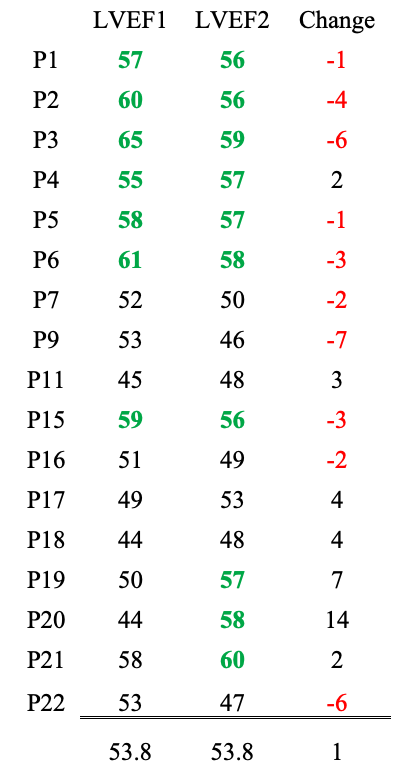
Barmada et al. evaluated RVEF and LVEF, which stand for right and left ventricle ejection fraction — i.e., the percent of the blood ejected from the right and left ventricles during contraction (systole).
Data from initial hospitalization and follow-up after at least two months right and left ventricle ejection fraction data are presented with the right ventricle ejection fraction on top and the left ventricle data on bottom (available for 17 of 23 patients).
Values for healthy young males should be >55% although this number varies from source to source. (https://doi.org/10.1161/CIRCIMAGING.113.000706 Circulation: Cardiovascular Imaging. 2013; 6:700–7)
From Barmada et al.
RVEF: Two patients had normal values at follow-up. Seven had lower EFs after “recovery,” and seven improved but were below 55% at follow-up.
LVEF: Seven patients were in the normal range during hospitalization and after at least two months. Three patients improved, and nine declined.
Late gadolinium enhancement (LGE) on cMRI is an indication of ongoing myocardial inflammation/fibrosis. (https://radiopaedia.org/articles/late-gadolinium-enhancement-2?lang=us) Data are available for 17 patients. LGE1 indicates results during hospitalization. LGE2 follow-up was performed at least two months later.

Fifteen of 17 patients had LGE in the acute phase of their illness and 14 of 17 began or continued to have LGE after their “recovery”. Three patients went from positive to negative and two went from negative to positive. The long-term damage to these hearts was not emphasized by Barmada et al. We cannot determine how many of these young people will be needing heart transplants, pacemakers, and other future treatment for their damaged hearts.
The CDC has known that myo/pericarditis occurs after C19 gene therapy as has been made public. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html)

The following data are from VAERS using myocarditis “signal” for all vaccines.
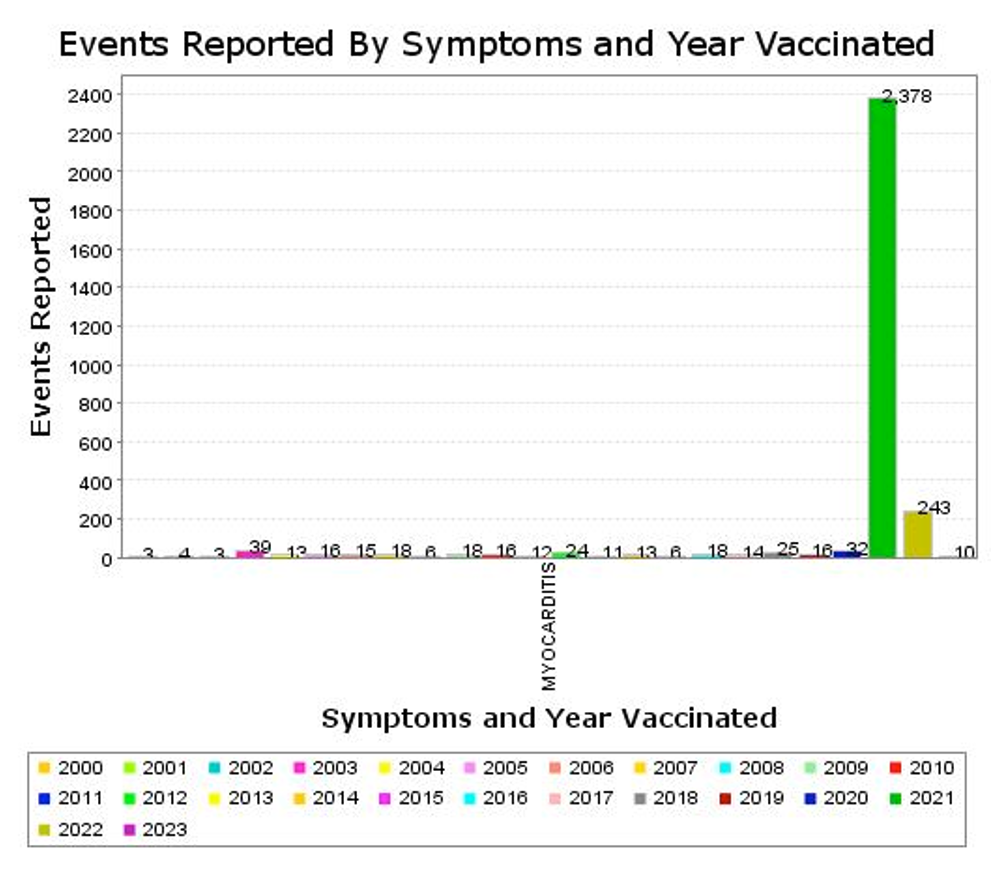
Of the 2,378 event reports in 2021, 2,345 were associated with C19 gene therapy products.
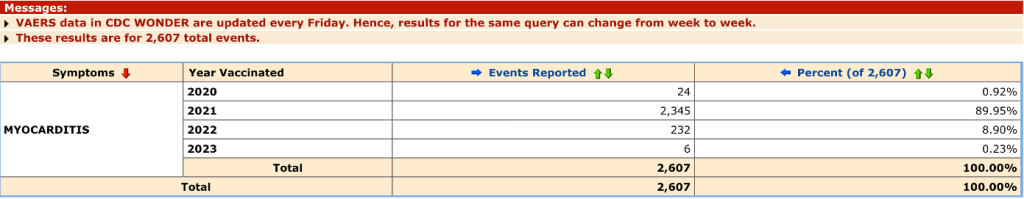
Males had 71.68% of these events, 21% occurred in ages 6 to 17, and 33% occurred in 18- to 29-year-olds.
As noted previously, VAERS numbers change over time, but this pattern is remarkably consistent with a low level of reporting in children under 18 years old in the VAERS database for 30 years including the COVID-19 year of 2020.
It was in 2021 that the number of myocarditis reports jumped, and then they fell back in 2022 as dosing with C19 “vaccines” tapered off. The mRNA products were authorized for 12 to 18-year-olds in May 2021 as the dosing program headed to its peak.
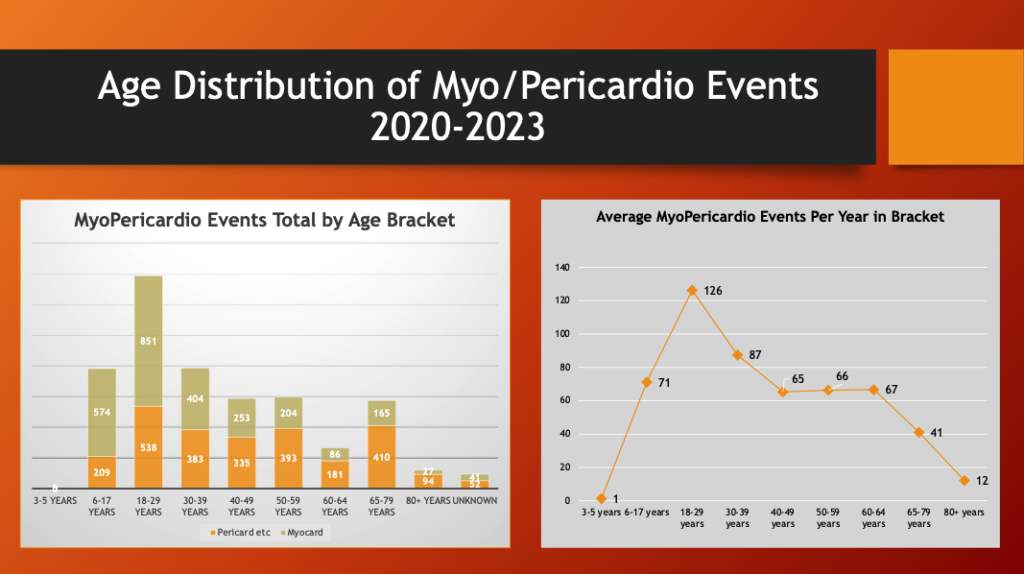
The left histogram above is a plot of the distribution of combined myo/pericardial event reports by age bracket with the average per year within the bracket on the right.
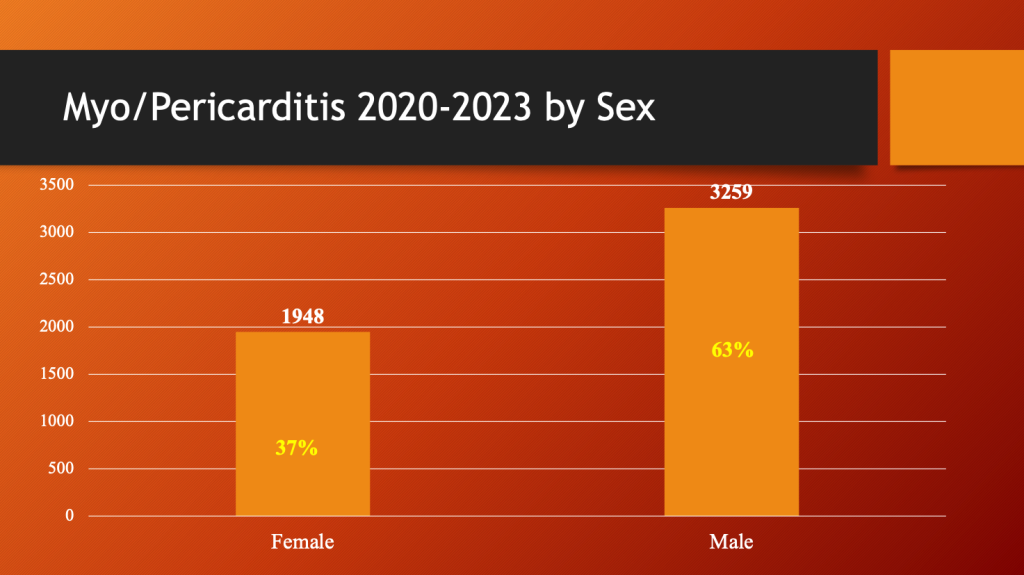
Males dominate the category of inflammatory cardiac disease.
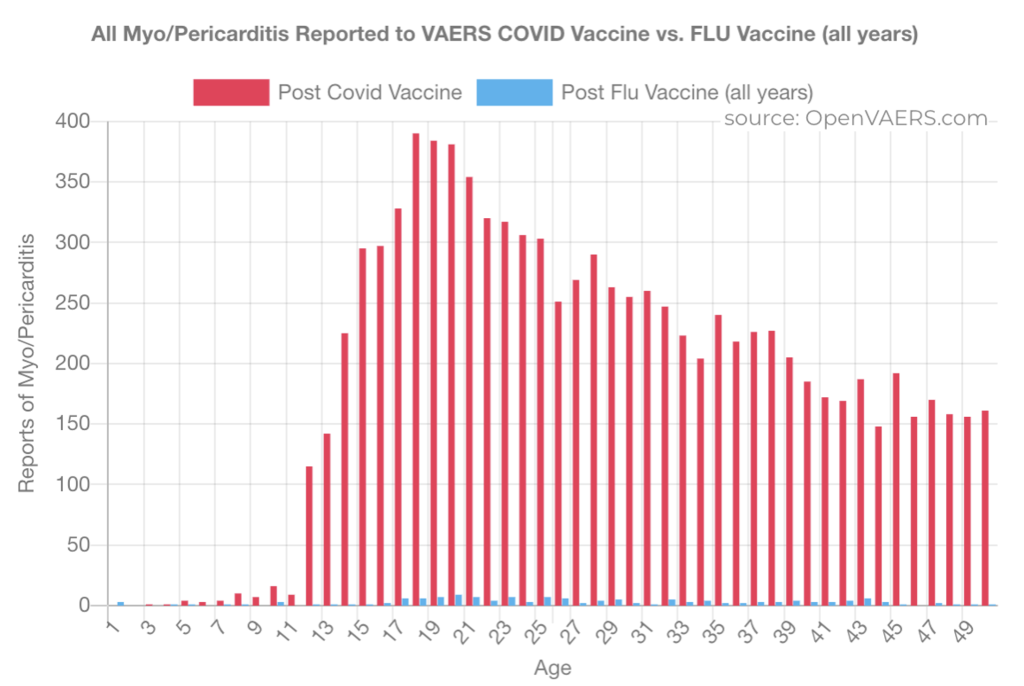
This chart from OpenVAERS shows the contrast between the reporting of myo/pericarditis after flu vaccine, in blue, compared with C19 gene therapy products, in red. Flu hardly shows up.
IV. Death, Sudden Death, and Sudden Cardiac Death
A. Tampering with VAERS Reports
Dr. Makis tracks morbidity and mortality reports in public media on his Substack. His June 10, 2023, article reports on the work of Alberto Benavidez who has been researching the veracity of VAERS for two years. (https://welcometheeagle.substack.com/p/vaers-jun-2-2023-wrap-up) Mr. Benavidez estimated the number of fatalities in children listed in VAERS should be increased by at least 182.
“There are at least 182 children who died from COVID-19 vaccines hidden in the VAERS database, that don’t show up when you search for child vaccine deaths.
How is VAERS doing this? It’s creative and diabolical:
Some brilliant investigative work was done by TheEagle88, who publishes his work on his Substack, and who made this shocking discovery (click here).”
https://makismd.substack.com/p/vaers-is-cleverly-hiding-182 child
In addition to mislabeling, TheEagle88 cites the efforts of Jessica Rose to track the disappearance of reports. The cases entered into the system can be removed as new ones are added. (https://jessicar.substack.com/p/the-death-counts-been-slowing-down, https://public.tableau.com/app/profile/alberto.benavidez.)
Mr. Benavidez says he has specialized training and experience in investigating billing and health insurance matters. He presents the following list of ways VAERS is being manipulated.

“Conclusion: VAERS is actively covering up catastrophic injury and to add insult, VAERS does not publish all legitimate reports received!”
Case 1:
VAERS ID 1952747, mentioned in TheEagle88’s reporting, is reproduced below. It shows the clinical detail submitted for a 12-year-old male captured by Mr. Benavidez before it disappeared. No age is indicated, yet the clinical detail identifies the young man’s age. (https://www.medalerts.org/vaersdb/findfield.php?IDNUMBER=1952747)
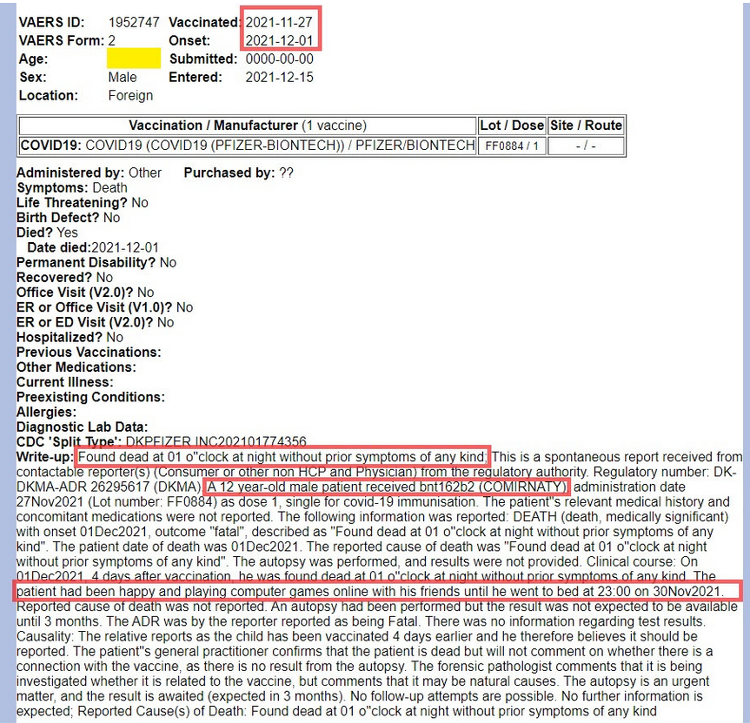
This entry for VAERS ID 1952747 is now blank. See below.
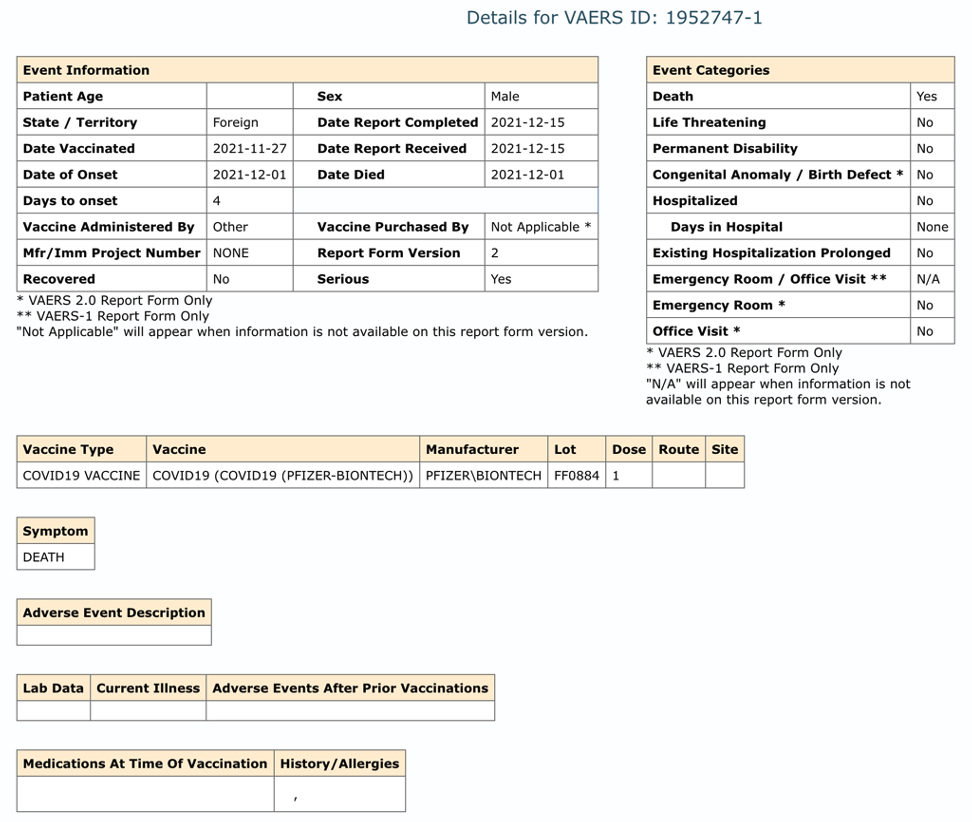
VAERS 6/10/2023
Case 2:
VAERS ID 1887456 concerns a two-year-old male who received one dose of BNT162b2 on November 18, 2021, seven months before the drug was released to his age group by Health and Human Services (HHS) Directive on June 18, 2022. Within six hours of receiving the injection, the child began hemorrhaging from his eyes, ears, nose, and mouth and then he died. The report was received November 20, 2021.
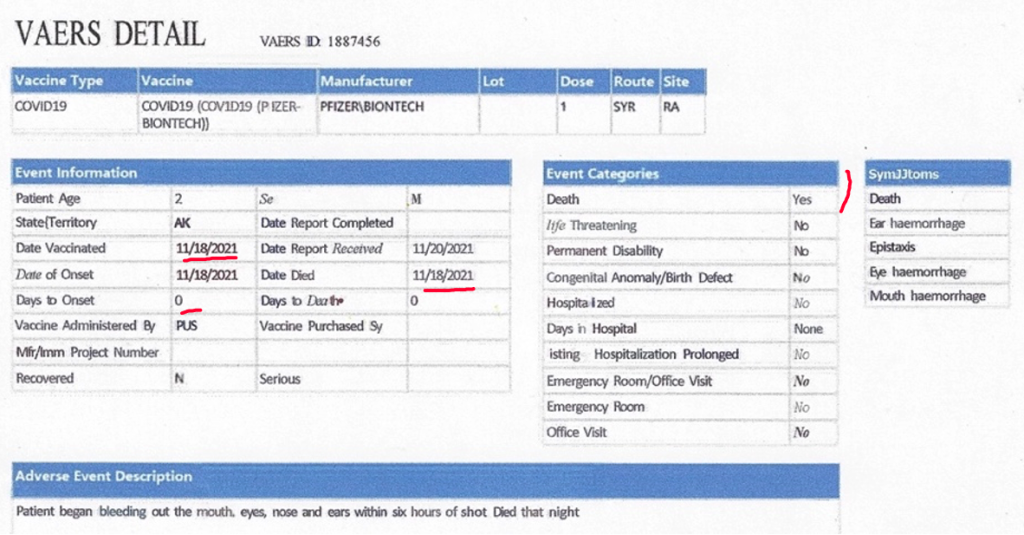
The report has since gone down the CDC memory hole as documented on June 12, 2023, below.
B. VAERS Data: Red Flag Alert
The following composite illustrates search results for “death” after “vaccination” in different VAERS age brackets.
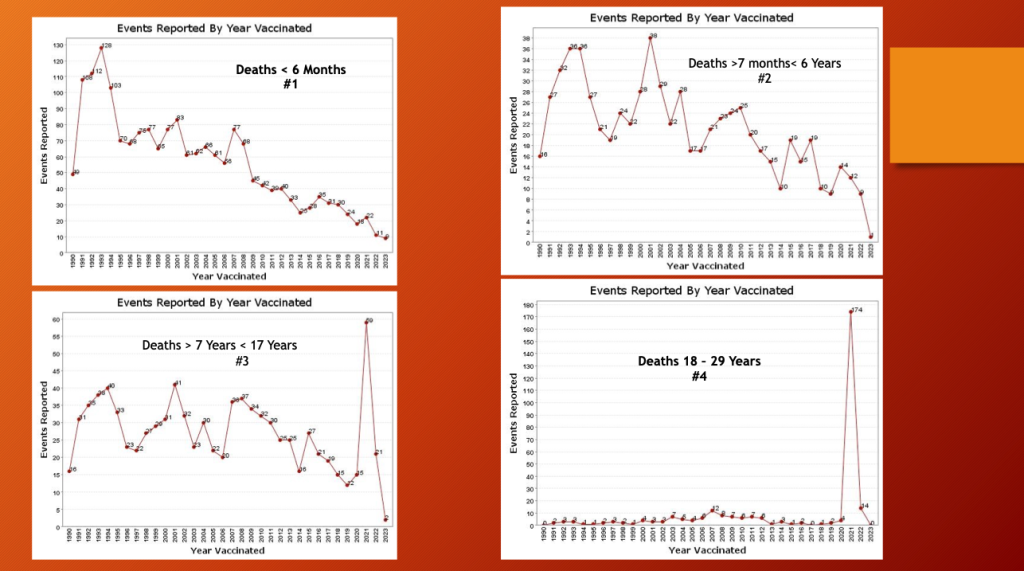
Charts #1 and #2 are reports of death in ages under seven years. Both plots show a 30-year downward trend in fatalities after vaccination. In 2021 there was a slight bump up in infant fatalities but within the range of past variation. The popularity of the injection waned before these children were included in the inoculation scheme.
Charts #3 and #4 show an entirely different pattern with a substantial “death” bump in 2021 with a 47x increase in fatalities for ages 7 to 17 years and a 43x increase in deaths in 2021 for ages 18 to 29 years.
The gene therapy products were authorized for ages 16 years and older on December 11, 2020, ages 12 to 15 years on May 10, 2021, and ages 5 to 11 on October 29, 2021. The age six months and older release date was June 17, 2022, supporting the hypothesis that these products played a role in the fatality spikes.
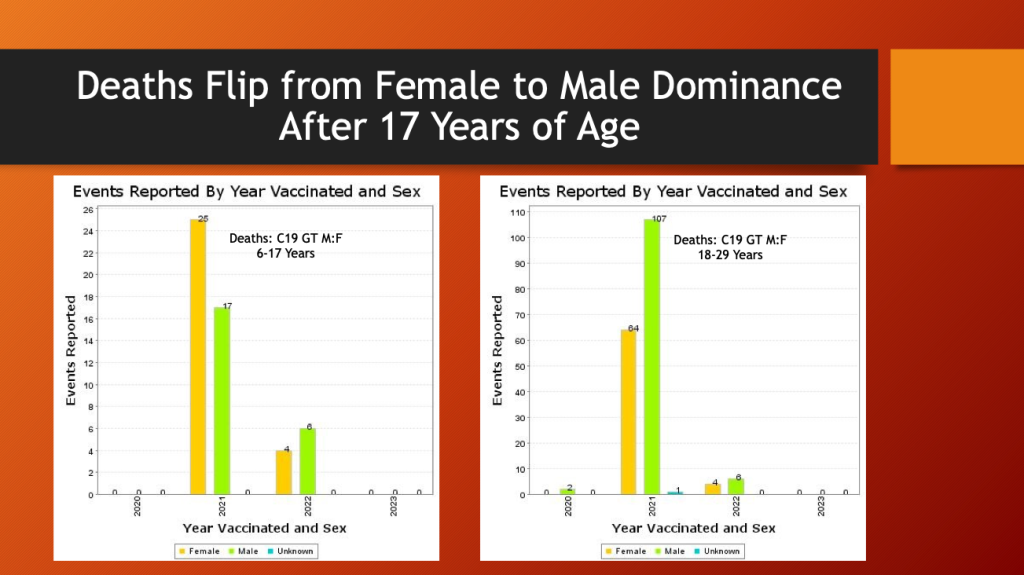
From 6 to 17 years of age, females had 60% of the fatalities contrasted with the next older age bracket, 18-29 years old, in which males had 63% of the fatalities. The total number went from 42 in the 6-17 years old bracket to 171 in the 18-29 years old bracket.
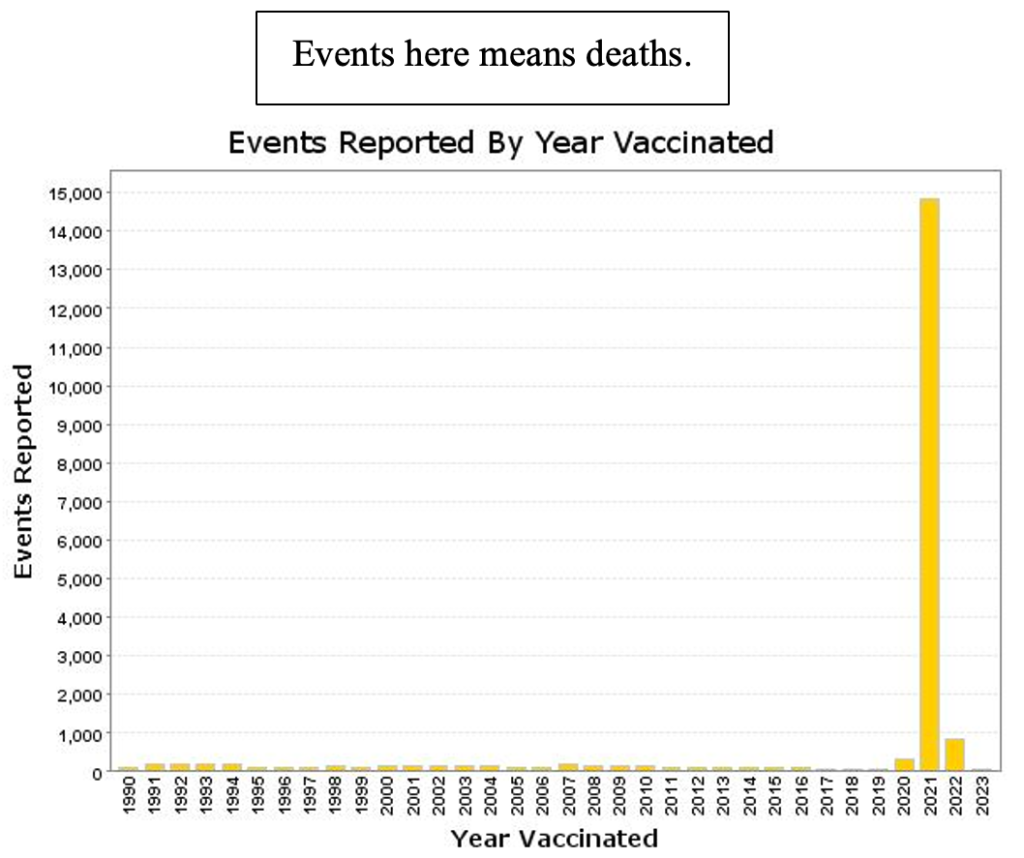
Total deaths (all ages) reported to VAERS for all “vaccine” products spiked in 2021, the year of the widescale C19 gene therapy products rollout.
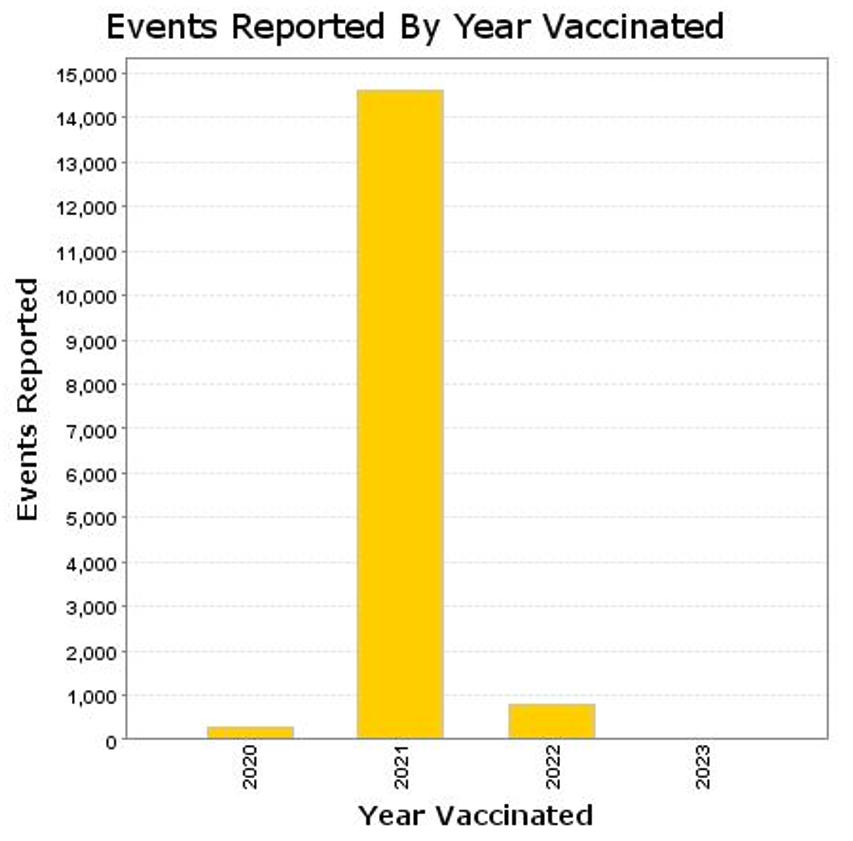
CoVax gene therapy products account for almost all deaths since the December 2020 emergency use authorization with very small adjustment for non-C19 drug products (compare this with preceding histogram).
VAERS ID 1913198 concerns a 13-year-old girl who experienced rapid onset of a very rare and aggressive epithelioid sarcoma of her heart one month after her first dose of BNT162b2.
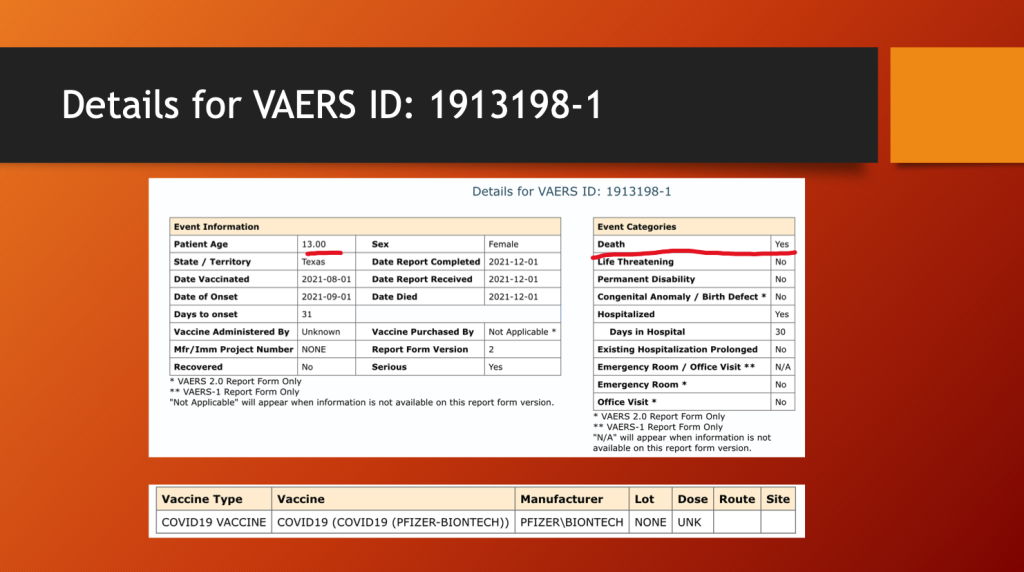
She spent 30 days in the hospital before she expired.

Her sarcoma recurred after excision and with chemotherapy.
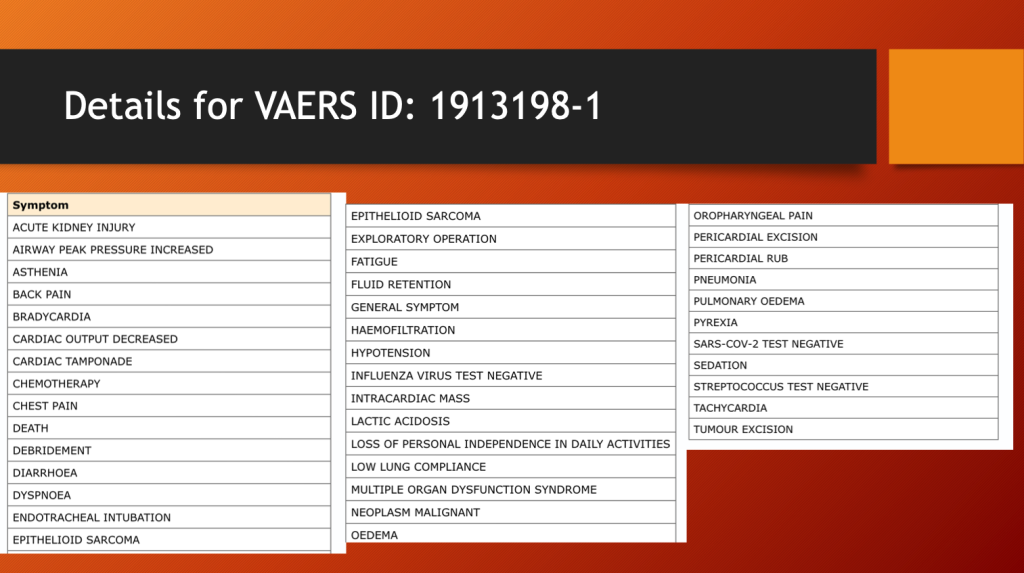
In medicine, this is called a problem list. This is a long one. She could be placed in multiple diagnostic categories including turbo cancer, cardiac epithelioid sarcoma, MIS-C, and myopericardial disease. For more on turbo cancer see: https://makismd.substack.com/p/turbo-cancer-sarcomas-14-yo-jeremiah, https://makismd.substack.com/p/turbo-colon-cancer-diagnosis-to-death, andhttps://makismd.substack.com/p/turbo-lung-cancer-24-year-old-uk.
VAERS ID 2576556 was a 13-year-old girl who died on Christmas Eve three weeks after her third dose of Pfizer’s BNT162b2.
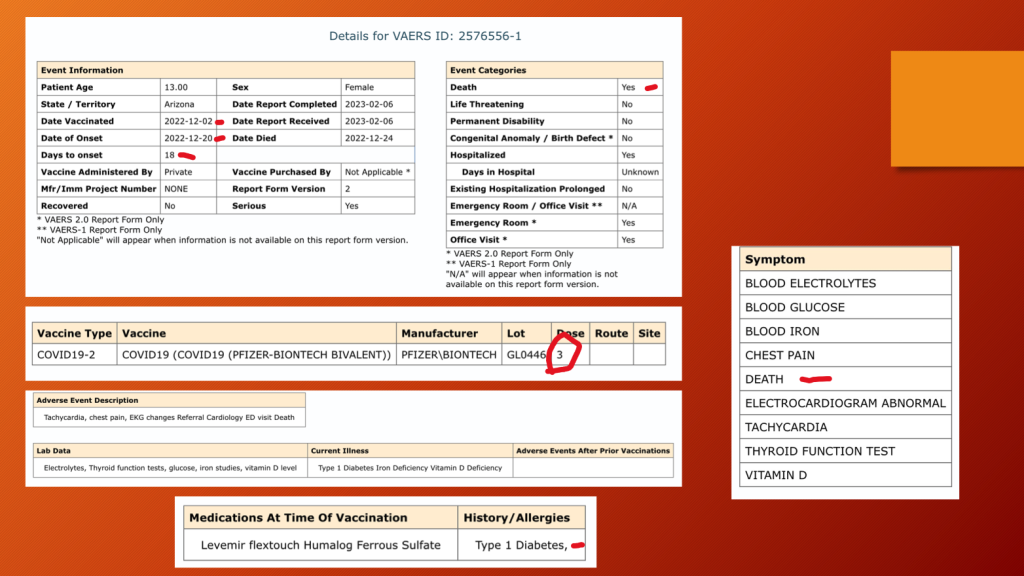
VAERS 5/12/202
This section will end with a case of what may someday be called fatal toxic vaccinosis:

Was the cause of death 11 doses of “vaccines”? We cannot say, as VAERS has limitations, but VAERS was touted as being a resource to monitor adverse event signals. Unfortunately, the signals are being ignored.
C: Excess Mortality
Ethical Sceptic has looked at all-cause mortality in the zero- to 24-year-old age bracket (below). There was a big jump in 2021, a five-sigma event, at the time the spike-generating drugs were being distributed widely. The years covered have unique importance with 2018 and 2019 predating C19 and offering a baseline, 2020 reflecting C19 alone; 2021 reflecting C19 plus C19 gene therapy product mass inoculation, and 2022 reflecting tapering of both C19 and C19 gene therapy. Mortality took a big jump in 2021 as the mass inoculation program launched.
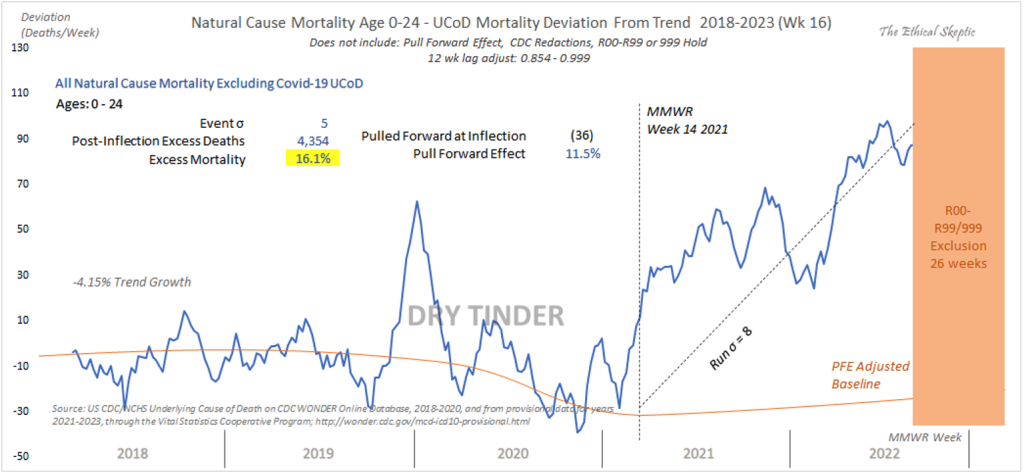
https://theethicalskeptic.com/2022/08/20/houston-we-have-a-problem-part-1-of-3/
https://theethicalskeptic.com/2022/10/24/houston-the-cdc-has-a-problem-part-2-of-3/
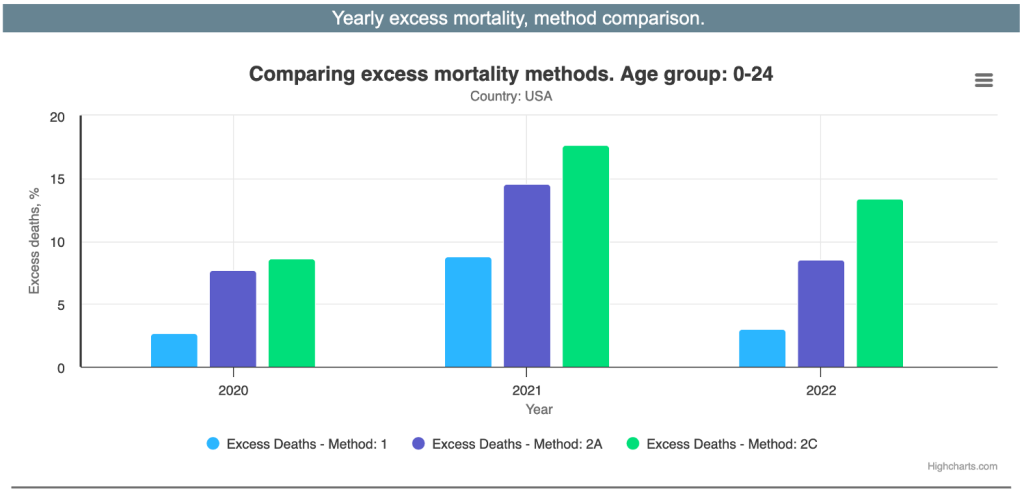
Ed Dowd and his group used three methods to estimate excess mortality in young people. (https://phinancetechnologies.com/HumanityProjects/Yearly%20Excess%20Death%20Rate%20Analysis%20-%20US.htm) The range was 2.7% to 8.6% in 2020, COVID-19 year one, and more than doubling in C19, “vaccine” year one, to 8.7% to 17.6% then dropping to 3% to 13% as the rate of “vaccination” dropped.
V. Adverse Events and Dosing Schedules: Correlation
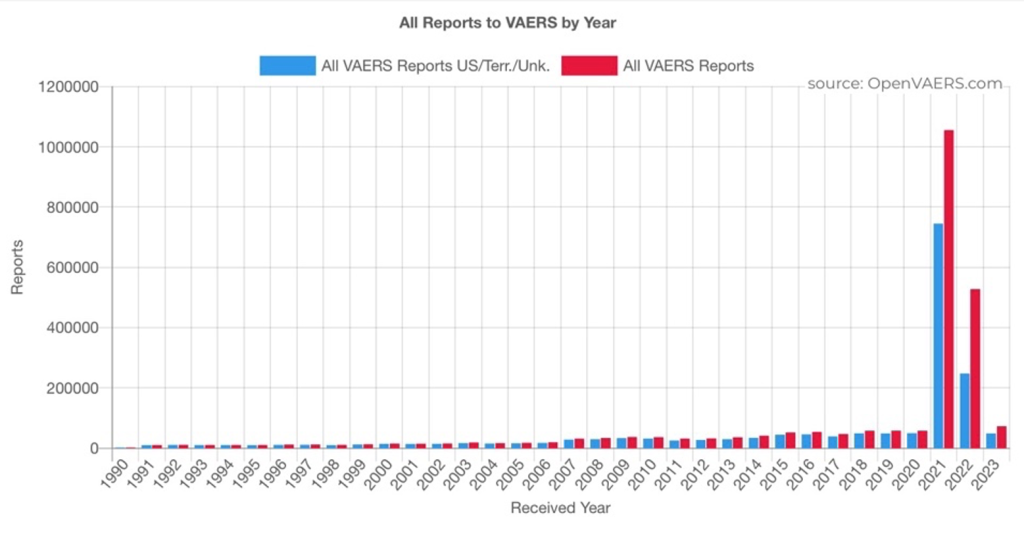
The histogram below is a plot of annual VAERS reports data since 1990. Years 2021 and 2022 demonstrate VAERS reporting spikes as the GTP “vaccine” program picked up steam. Then, it began cooling off in 2022. From Pfizer Document 2.4 (reporting on animal studies in the early phases of development beginning in early 2020) forward, there has been documentation of dose-related adverse events associated with the LNP/mRNA products. (https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf)
The almost two-and-a-half years of data support this observation. The more drug administered, the more complications.
Is this hypothesis supported with individual disease categories?
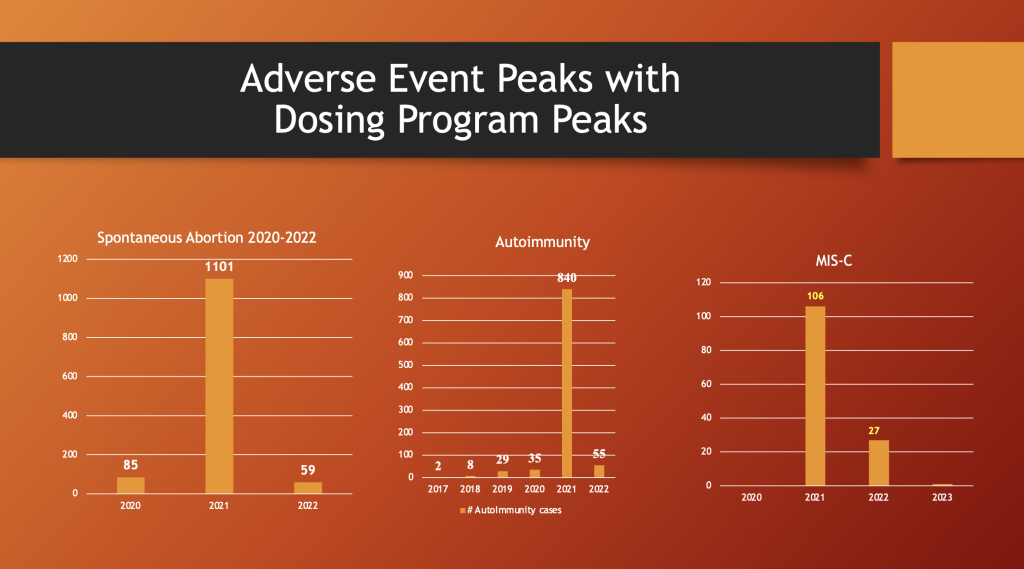
VAERS 5/12/2023
Spontaneous abortion, autoimmunity, and MIS-C patterns peaked in 2021 along with the peak in drug dosing.
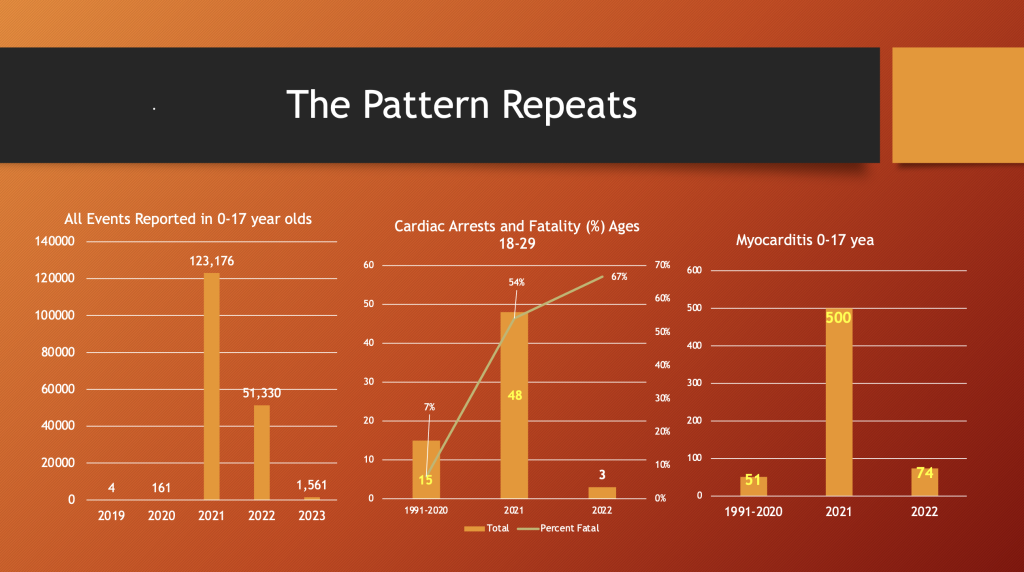
VAERS 5/12/2023
The same is true for all events (ages 0-17 years), cardiac arrests (ages 18-29 years), and myocarditis (0-17 years).
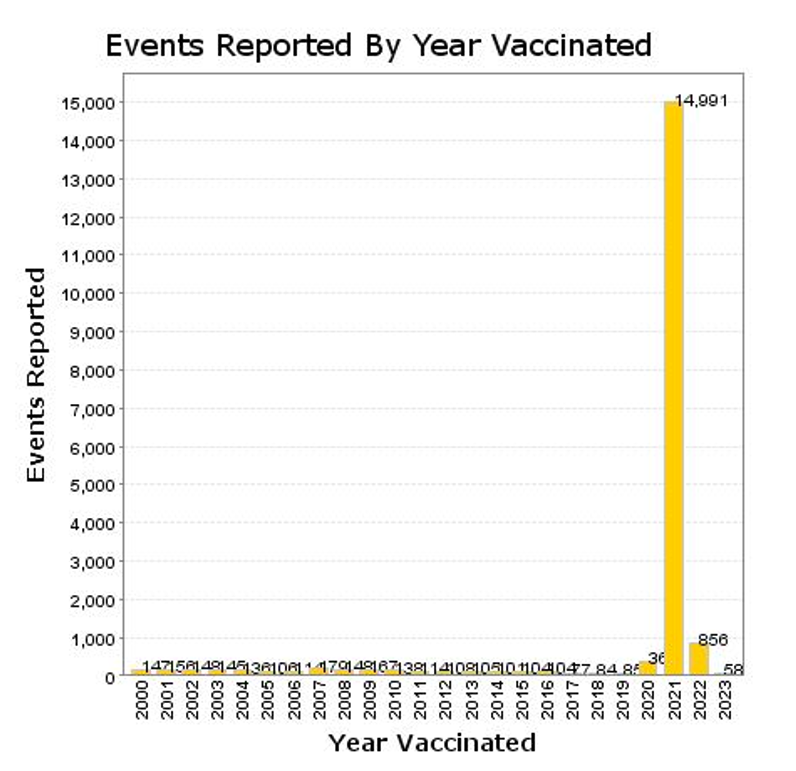
The plot above illustrates the enormous jump in mortality reporting events in VAERS in 2021, the year the gene therapy products were rolled out.
The next chart is a plot of daily doses administered in the U.S. and Territories. Note the primary peak in dosing during spring 2021 with a secondary peak beginning in late summer of 2021 and extending through January 2022. Two additional peaks are present.
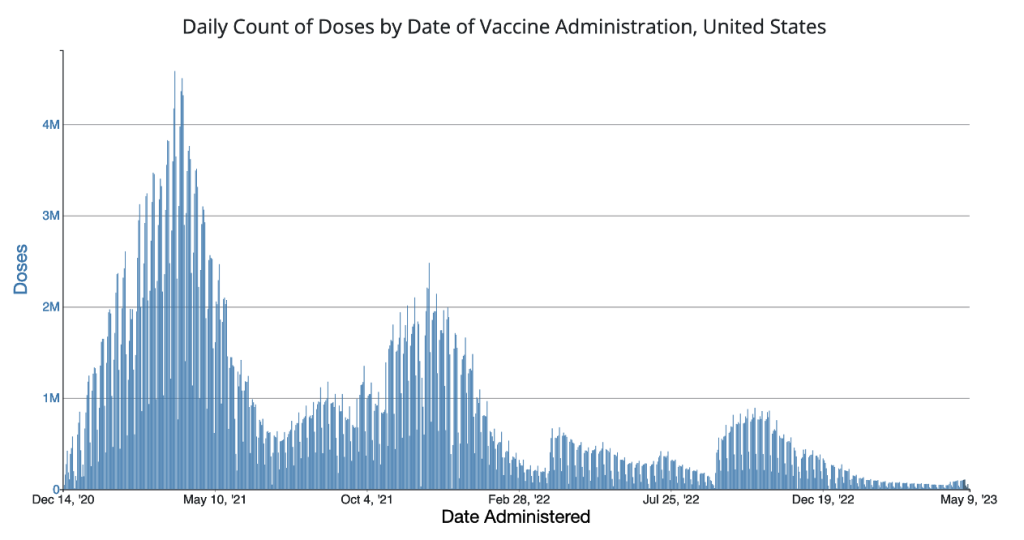
https://covid.cdc.gov/covid-data-tracker/#vaccination-trends
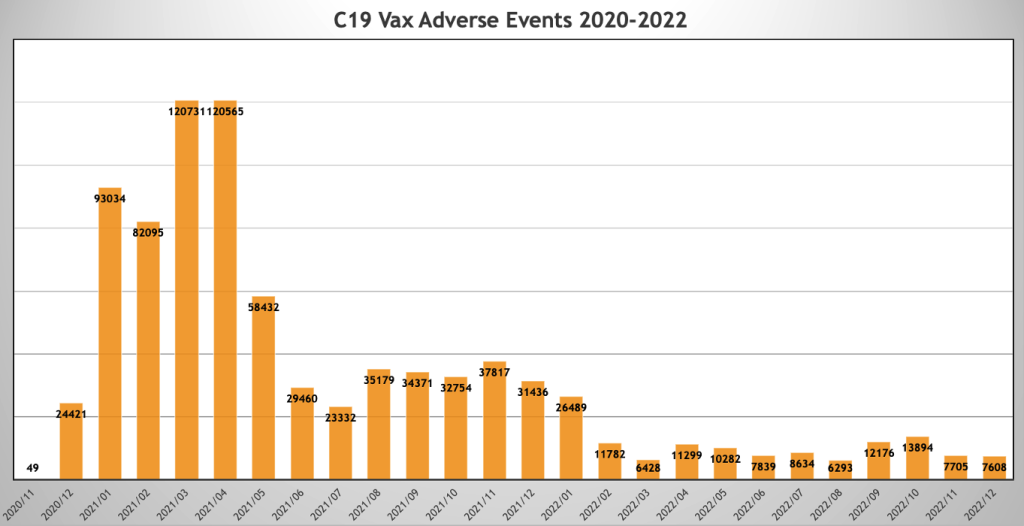
VAERS event reports followed a similar pattern with peaks in February and March 2021 and a secondary peak in August 2021 through January 2022.
Below is a plot of monthly people injected on the primary axis in orange, and monthly VAERS Event Reports are plotted on the secondary axis (scaled for illustration purposes).
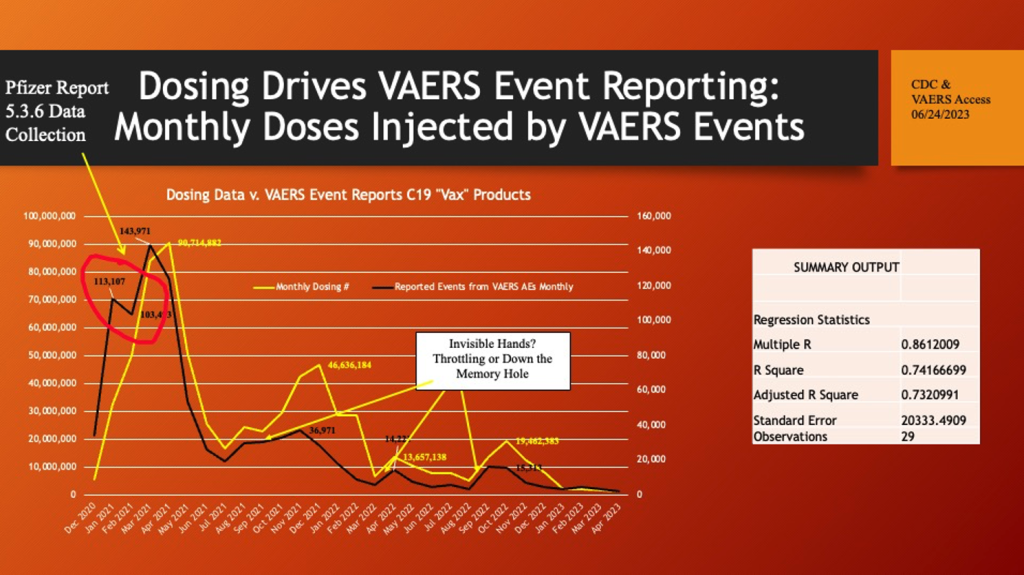
Data are restricted to U.S. and Territories. Data Accessed 06/24/2023.
There is a strong association between dosing and adverse events.
What accounts for the abrupt decline (red circle) in reporting of events in February 2021? At this point in time, the data collecting for Pfizer Confidential Post-Marketing Report 5.3.6 was in the late stage of its compilation. Pfizer had to add 2,400 employees to record over 140,000 adverse events during the first 10 weeks of widespread use of C19 gene therapy drugs. Did the CDC throttle back posting of events? What are the alternative explanations of this anomaly? Did they throttle down the next three Event Report waves as well?
Superimposing monthly C19 gene therapy drug monthly doses (orange) on a plot of VAERS reports by month of injection (blue) shows a similar peak and valley pattern. When combined with the other plots, these are evidence of a possible causal relationship between the number of monthly doses and the number of adverse reports in the VAERS database.
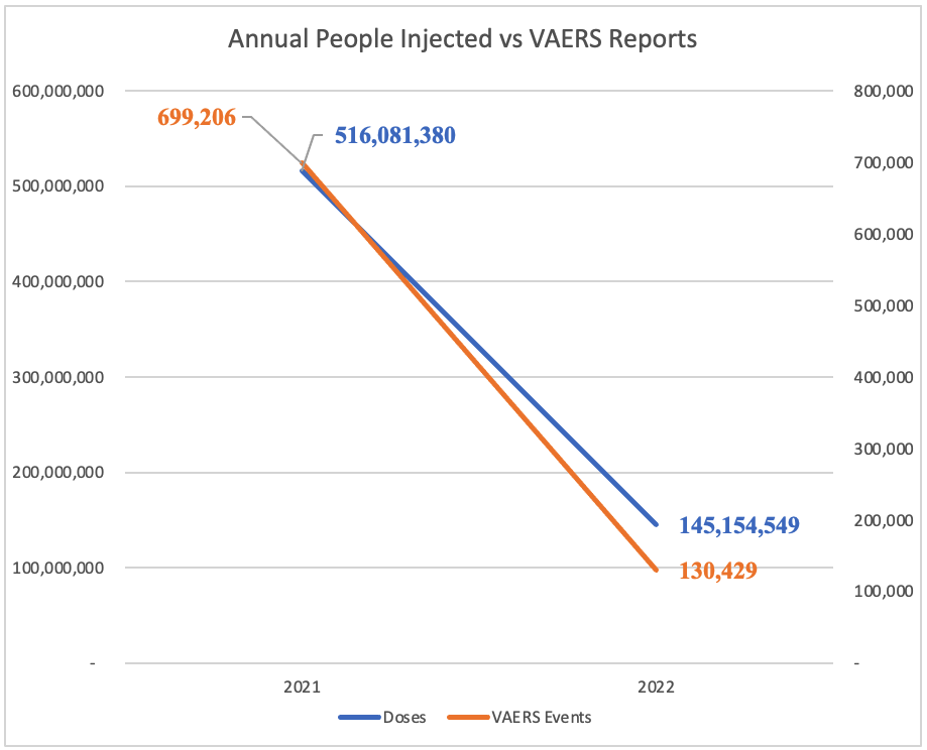
The reduced amplitude of the secondary, tertiary, and quaternary peaks of reported events related to the dosing peaks are possibly explained by blanks and reformulations of the C19 gene therapy product or meddling with the numbers. Efficacy proved elusive, but toxicity could be reduced with removal of mRNA from some batches and reformulations of the “recipe.” (https://dailysceptic.org/2023/06/28/pfizer-vaccine-batches-in-the-eu-were-placebos-say-scientists/) As dosing fell off in 2022, VAERS reporting fell in tandem (below).
VI. Discussion
The SARS-CoV-2 virus emerged from a laboratory and spread around the world wreaking havoc in many ways. Some havoc was related to illness caused by Dr. Ralph Baric’s chimera but other than the elderly and those with certain co-morbidities, the virus posed little threat to healthy, young people. Recently, a Freedom of Information (FOI) request to the Ministry of Health in Israel asking for documentation of any deaths of persons less than 50 years of age without comorbidity brought the response, there were none. (https://www.illusionconsensus.com/p/new-israeli-report-no-covid-deaths)
As Dr. Ioannidis et al. recently reported, COVID-19 is an illness of Seniors.
“The COVID-19 death risk in people <65 years old during the period of fatalities from the epidemic was equivalent to the death risk from driving between 13 and 101 miles per day for 11 countries and 6 states, and was higher (equivalent to the death risk from driving 143-668 miles per day) for 6 other states and the UK. People <65 years old without underlying predisposing conditions accounted for only 0.7-2.6% of all COVID-19 deaths (data available from France, Italy, Netherlands, Sweden, Georgia, and New York City).” (https://doi.org/10.1101/2020.04.05.20054361)
In response to COVID-19, an experimental gene therapy product was labeled a vaccine and, with massive funding for marketing from the U.S. government, was foisted on the peoples of the world. Like the virus itself, the new drugs had a negative impact on Seniors; but, unlike SARS-CoV-2, has had devastating effects on those less than 60 years old. Children with miniscule risk from the virus have died and been disabled by the spike-producing gene therapy drugs.
Beginning before conception, these drugs have negatively impacted reproduction. Women are disproportionately affected with adverse events and disability. Young men are disproportionately afflicted with heart damage, which may be permanent for many. Unlike the virus, the treatment of the virus has impacted all age groups, including children, healthy people, as well as those with no comorbidities.
We do not yet know whether these new drugs and the diseases that they cause will go away or are now part of the human condition. If, a big if, they are integrated into the human genome and transcribe and translate abnormal proteins, these new disease conditions may be propagated to future generations. Natural selection has never been set up against synthetic genetic materials and the host of new diseases produced by them.
Perhaps even more disturbing is the work of Kevin McKernan who identified bacterial DNA from the manufacturing process present in the “vaccines” at a level 1,000 times above the acceptable level. (https://sashalatypova.substack.com/p/kevin-mckernan-reports-on-plasmidgate) This bacterial DNA has unknown consequence at present but has the potential of producing resistance to an important class of antibiotics, the aminoglycosides.
For now, these negative thoughts are not supported by known science. The robustness and complexity of the human immune system may yet defeat this threat. Once governments around the world stop the coverup of these various man-made C19 gene therapy-related illnesses, doctors and researchers can begin the task of helping the injured and finding cures for their diseases.
Acknowledgements
Amy Kelly, COO of DailyClout.io and Project Director of the War Room/DailyClout Pfizer Documents Analysis Project, and Linnea Wahl, M.S., War Room/DailyClout Pfizer Documents Team Five Leader, provided invaluable editorial assistance in preparing this manuscript. Ed Clark and Tony Damian supplied data where noted, and Rudy Parrish critically reviewed the statistical analysis.
No comments:
Post a Comment