The FDA's War Against America's Health
How the FDA habitually buries life-changing natural medicines and relentlessly props up unsafe and ineffective pharmaceuticals
Story at a Glance:
•In 1906, the first FDA (Food and Drug Administration) was created in response to massive public protests against adulterated food and drugs (e.g., rotting food partially preserved with food additives or counterfeit consumer products). To stop it, food industry lobbyists attempted every tactic imaginable, eventually taking over the Department of Agriculture and in time forcing the dedicated public servant leading it (Harvey Wiley) to quit. Because of this, many toxic food additives Wiley fought against gained “generally recognized as safe” (GRAS) status and remain in use today.
•The handicapping of the FDA came to a head in 1962, when thalidomide was just barely prevented from devastating America’s children, prompting Congress to give the FDA much broader powers to police the safety and efficacy of drugs.
•Unfortunately, this law backfired, as the FDA created an impossible to reach standard of efficacy that it selectively enforced to protect the pharmaceutical industry and simultaneously began utilizing increasingly brazen (and illegal) police tactics against anyone promoting effective natural therapies.
•Because of this, many life-changing medical therapies (discussed throughout this article) were blacklisted by the FDA and faded into obscurity. This, along with the FDA’s tendency to push unsafe and ineffective therapies (e.g., vaccines or antidepressants) onto the market regardless of how much data argued against doing so, led to the FDA becoming the most protested agency in the federal government.
•Nonetheless, every attempt to fix the FDA failed. In this article, I will review the structural issues that have perpetually caused the FDA to succumb to incompetence and corruption. I propose potential solutions that can utilize the unprecedented window created by the Make America Healthy Again movement to end the FDA’s war against America’s health.
For most of my life, I have observed the FDA belligerently suppress natural treatments and any unorthodox therapy which threatens the medical monopoly while simultaneously railroading through a variety of unsafe and ineffective drugs regardless of how much public protest the agency meets. Consider for example, this 2004 Senate testimony by the FDA scientist who got Vioxx banned that accurately described exactly what would come to pass with the COVID vaccines two decades later:
As
such, I do not hold the FDA in a positive light, especially given that
during COVID-19, I (like many others) spent hundreds of hours trying to
get the agency to allow the limited use of off-patent therapeutics for
COVID-19—all of which ultimately went nowhere due to the unjustifiable
roadblocks the agency kept putting up.
Note: given my
familiarity with the FDA’s conduct, I felt the odds were against those
endeavors succeeding, but I nonetheless exhausted myself supporting
effective alternative therapies because I didn’t want to live with the
knowledge I could have done something that could have prevented the
unfolding tragedy but chose not to.
Over the last year, and especially since Trump won the election, I have received a lot of inquiries as to how the FDA could be reformed over the next four years. Given the importance of presenting the issue correctly, I’ve spent a lot of time trying to look at both sides of the question.
In medicine, “sensitivity” denotes how likely a test is not to miss something that’s there, while “specificity” denotes how likely a test is not to have a false positive. The great challenge with these two concepts is that there’s almost always a trade-off. Hence, as you increase one, the other reciprocally declines (e.g., as the PCR cycle threshold was increased on COVID tests, while it was harder to miss an infection, you also became more and more likely to get false positives from the tests—which is frequently what happened). In turn, many issues in medicine result from a poor balance between the two (e.g., Peter C. Gøtzsche made a good case that the sensitivity for routine breast cancer screening is too high, which leads to many women being erroneously diagnosed with dangerous breast cancers and subject to unnecessary treatments).
This same trade-off also exists throughout politics as there are often two conflicting positions (e.g., wanting a robust death penalty to serve as a deterrent against violent crimes but also not wanting to execute innocent individuals), and in many cases the eventual position that’s settled on is the result of a prolonged battle that eventually reaches a midpoint between sensitivity and specificity that while not ideal, is begrudgingly acceptable to both sides. In my eyes, the most important thing to understand about this dynamic is that it typically takes an incredible amount of work to reach the functional compromise that’s eventually settled upon, so if the existing process is scrapped (e.g. because people who are polarized on the issue can only see it from their side’s perspective) what follows (e.g., a complete lack of police enforcement in high crime areas) is often much worse than what preceded it.
In the case of the FDA, the agency’s situation has run into a similar issue—the FDA is expected to keep bad foods and drugs off the market while not blocking good ones from getting to the public. While this seems “simple,” it’s actually an incredibly challenging task, and the agency's history is one of it frequently abysmally failing at both—even when its leadership was composed of dedicated public servants who put the wellbeing of the American people before everything else.
Crime Against the Food Law
In
the late 1800s, food producers would constantly sell adulterated food,
while early pharmaceutical companies would sell a variety of proprietary
medicines with secret ingredients that were inevitably things like
opium and alcohol. Gradually, public outrage built around this,
particularly since journalists and newspapers were willing to expose the
issue (e.g., Upton Sinclair’s 1904 book The Jungle played a pivotal role in awakening the public to the immense problems with the meat industry).
Eventually, in 1906, the Pure Food and Drug Act
was passed, which gave the Bureau of Chemistry the authority to ensure
products sold in America were accurately labeled (e.g., no hidden opium
or counterfeit foods), and that the food was not adulterated.
While the publicly strongly supported this law (e.g., the public did not want to eat potentially dangerous food additives), the industry resisted and relentlessly fought Congress not to pass the law, using many of the same lines and ploys we would see today (e.g., industry lobbies would always appear to shut down any attempts to legislate against this). Eventually, it passed with the compromise that the courts would be the means to challenge enforcement actions by the law.
The director of the Bureau of Chemistry (and thus the first head of the FDA), Harvey Wiley, felt very strongly about the dangers of chemical additives being put into our foods and in 1905, began a series of tests where he gave young healthy government volunteers (e.g., those most resistant to chronic poisoning) higher doses of the additives commonly being used in foods and was able to demonstrate the recipients gradually became ill.
Now, I want to say this, because I regard it as important. For fifteen or twenty days, or even longer in some cases, no visible effects were produced in what you would call "symptoms." The young men had normal appetites and performed their work without any discomfort, and had no complaints. After that time they began to eat their ration with some little discomfort. They were under obligation to do it, but they often said: "I wish you could let this go ; I don't want it." Their appetites began to fail. At the end every one of their appetites was very badly affected, and some of them were unable any longer to eat the full amount. Of course we never required anything that was impossible. They developed persistent headaches in most cases, followed by general depression and debility. It was extremely well marked in every instance.
It had a worse effect in the food when they knew it was in the food, because it became repugnant to them.
Unfortunately the effects in some cases were very much prolonged. Some of the young men—the experiments ended in July, or in June, the end of the year—and some of the young men complained even through the summer, and it was late in the autumn before they recovered their full normal appetites.
Note: the additives Wiley tested were boric acid and borax, salicylic acid (aspirin) and salicylates, benzoic acid and benzoates, sulfur dioxide and sulfites, formaldehyde, sulfate of copper (used to green produce), and saltpeter (nitrates).
In turn, a schism gradually developed in the scientific community, where Wiley (and many other respected doctors and physiologists) argued evidence showed those additives were dangerous. At the same time, a variety of scientists (who were paid off by the food industry) misleadingly testified to both Congress and then later the courts that the additives were “safe” or necessary (e.g., to prevent microbial food poisoning).
He came up and introduced himself to me [Wiley] and attempted to make some apology for his part in the activities of the Remsen Board [which was created to sabotage the FDA]. He realized very keenly the condition they were in, in espousing the cause of adulteration, becoming the paid agents of the adulterators, and incurring the universal condemnation of the press and the people of the country. Dr. Herter was then a very sick man. In a few months from that date he died.
Initially, the honest scientists (fully backed by the public) won, and Congress gave the Bureau of Chemistry the full authority to clean up the food. Still, the industry was relentless, and after failing in the courts (even in friendly jurisdictions), decided to target the executive branch directly, and successfully convinced the Secretary of Agriculture to sabotage Wiley’s work. At first, President Theodore Roosevelt vigorously opposed these efforts, and protected Wiley, but eventually he sided with the industry, and created a board (not authorized by the 1906 Food and Drug Act), which overrode everything Wiley tried to do.
Roosevelt’s about-face in turn, occurred for four key reasons.
•The
food law that was passed was different from what Roosevelt had
initially wanted (he wanted it to focus on meat, but the eventual meat
provision that was added at the very end differed was considerably
altered).
•He experienced an increasing number of complaints that the Food and Drug Act was costing industry and trading partners money.
•The
Secretary of Agriculture forced Wiley to testify against Roosevelt’s
position on importation taxes for Cuban sugar in front of Congress
(which greatly offended Roosevelt).
•When Roosevelt was alerted to the fact Wiley wanted to remove saccharin from the marketplace (Roosevelt’s favorite sweetener), this exchange took place (which due to its consequences, tormented Wiley for decades):
This answer was the basis for the complete paralysis of the Food Law. Turning to me in sudden anger the President changed from Dr. Jekyll to Mr. Hyde, and said: "You tell me that saccharin is injurious to health?" I said, "Yes, Mr. President, I do tell you that." He replied, "Dr. Rixey gives it to me every day." I answered, "Mr. President, he probably thinks you may be threatened with diabetes." To this he retorted, "Anybody who says saccharin is injurious to health is an idiot"
Note: I share this passage to illustrate how things that go catastrophically awry can often result from one unfortunate domino rippling out over decades.
In turn, while courts, state governments, legislatures, and most importantly the public supported what Wiley wanted to do, key parts of the executive branch did not. As such, his agency’s enforcement ability continually declined (e.g., virtually no enforcement actions were allowed to be brought against the thousands of cases of food adulteration they encountered), his inconvenient scientific research (e.g., on the dangers of arsenic, food colorings and preservatives in foods) was blocked from publication, partially successful attempts were made to frame him and evict him from his post (along with other types of retaliation occurring against other government employees who tried to fight for clean food), and once Taft became president in 1909, it became even more challenging for Wiley to enforce the laws (e.g., Taft overturned the ban on selling fake whiskey).
Note: in parallel to this, the Department of Agriculture created a “Bureau of Soil,” which usurped the Bureau of Chemistry’s responsibility for analyzing soil around the country (but ultimately never got anything meaningful done). This was highly problematic as it both handicapped the Bureau of Chemistry’s ability to do research, but also removed the systematic analysis of the chemistry of the nation’s soils (which was/is necessary as the trace minerals present make a considerable impact on the health of the plants and those who eat them). Likewise, another agency (the Bureau of Standards) decided it wanted to expand its influence and partnered with industry to create a variety of profitable technologies (that lay outside its Congressional mandate) while simultaneously usurping the Bureau of Chemistry’s resources and responsibilities to advance its own interests.
Eventually, in 1912, Wiley, one of the most respected public servants in the country, resigned because he realized he could do more to help the public as a private citizen than within the government and in 1927, the Bureau of Chemistry was turned into the FDA (at which point it lost the ability to do many of the critical functions it had provided to monitor the safety of the country). Far later, Wiley wrote the book “The History of A Crime Against The Food Law” (which can be read here and details much of the same abhorrent behavior we see now happening over a hundred years ago). To quote one newspaper from the time:
He [Dr. Wiley] has been practically without power to put the law into effect against strong offenders. He has been humiliated by being overruled by his subordinates. He has suffered from an inefficient administration of the Department of which his bureau is a part; for the venerable Secretary of Agriculture is too old vigorously to administer his great Department. Yet Dr. Wiley, purely for patriotic reasons, has suffered this hindrance and humiliation till some change might come which should unshackle him. On the outside the bad food and drug interests—or some of them—have maintained a lobby in Washington, have kept "syndicate" newspaper writers in their pay to write about the unfairness and the injustice of the law and the unreasonableness and "crankiness" of Dr. Wiley. One such organization—or pretended organization—some time ago sent a threatening letter to all the most important periodicals, saying that large advertisers would withdraw their patronage if they published articles favorable to the law!
To illustrate how much things remain the same, a series of investigative reports have recently shown that the lobbyists from the processed food industry are now working fervently behind the scenes to block RFK’s nomination and prevent him from reforming the industry as Secretary of HHS. Beyond the tactics being remarkably similar to what Wiley detailed the industry doing over a century ago, they also touch on a central point Wiley raised—the only way to create change in this industry is to coax the public at large to demand it, as the moment you rely upon the members of the government to fix it, lobbyists will crush those efforts. In turn, had RFK not created the Make America Healthy Again Movement and been very strategic in how he leveraged its clout, we’d never have a chance of cleaning up the food supply.
Generally Recognized as “Safe”
Many of the additives in our foods are “generally recognized as safe” (GRAS) and as such, very little regulation exists regarding their widespread use, regardless of their toxicity.
At the time Wiley was trying to correct the nation’s food supply, he faced two primary issues.
The first issue was that the industry constantly made counterfeit foods to save money. For example, one of the major controversies Wiley dealt with (that Roosevelt backed him on) was people trying to make fake whiskey by taking grain alcohol (which was much cheaper to produce than whisky) and then flavoring it to taste like whiskey and labeling it as such (which Roosevelt did not approve of). Another was that oyster farmers would often float oysters in (polluted) waters to make them bulk up, a practice quite similar to how many meats now are injected with water to increase their weight, while a third was that small fish which were not sardines were being widely sold as such.
The second issue was that he (and many individual states) tried to outlaw many of the more problematic additives put into our foods. Unfortunately, due to the FDA getting corrupted from the inside and the Supreme Court eventually siding with the food manufacturers, those additives were propped up despite widespread opposition to them, eventually achieving GRAS status, and since then have been widespread in our food supply.
Note: those additives included sodium benzoate, sulfur dioxide, alum (potassium aluminum sulfate), sulfur dioxide, saccharin, modified corn sugars (which preceded high fructose corn syrup), saccharin, and nitrogen bleached flour—many of which were linked to cancer. Sadly, since 2000, nearly 99 percent of new food chemicals added to the food supply chain have exploited the GRAS loophole. Of these additives, I personally believe the widespread use of aluminum in processed foods is likely the most detrimental (due to it greatly impairing the physiologic zeta potential and causing micro-clotting throughout the body), and provides a key explanation for why you often see certain rapid improvements in individuals once they stop eating processed foods.
Wiley
in turn, argued that if these additives could cause acute toxicity at
higher doses, they likely caused chronic toxicity and accelerated aging
at lower doses (yet remarkably paid-off scientists were able to argue
that it was acceptable to give food additives at ranges just below their
recognized toxic doses). Unfortunately, as the FDA became progressively
more corrupt, Wiley’s arguments became forgotten, and these “safe”
additives have chronically poisoned our society.
Note: a
key point Wiley emphasized is that unhealthy conditions would cause
organs to hypertrophy (enlarge) and lose their function—which was
especially consequential once the organs responsible for detoxification began to fail.
One of the most remarkable things about this debacle was that the food industry would continually insist that the “safe” additives urgently needed to remain legal, as without them, producing their foods would no longer be possible. Yet, in case after case (e.g., sodium benzoate preserving ketchup or sulfur dioxide for preserving rotting fruit), competing companies arose that could produce the foods without the toxic additives. More importantly, they successfully argued that the primary purpose of the additives was to make it possible for an inferior quality (adulterated) product to be still sellable (e.g. by preserving something that was rotting like moldy grains or artificially coloring something that had visibly lost its nutritional value). In many cases, the companies actually switched to not using the additives because the products without them were of higher quality and, thus more popular with consumers.
“Are you Dr. Wiley?” I said I was. He said: "A few years ago I was the president of the Long Island Oyster Association. We regarded you as the arch-enemy of our industry when under your direction the ruling was issued that we should not add water to oysters that we shipped, nor place ice in contact with the oysters that we shipped. We considered you a devil incarnate. Now we know that decision was the salvation of our industry and I want to take your hand and congratulate you on doing the greatest service to the oyster industry that could possibly have been done. We are selling a dozen times as many oysters now in a perfect condition as they come from the water as we did at the time of your ruling."
The Williams Brothers Company later came to believe that benzoate, or any other preservative was entirely unnecessary in such food products as ketchup, sweet pickles, preserves, etc., and then withdrew as a party to the suit [that fought to keep benzoate in use]. Not only did Williams Brothers find that a preservative such as benzoate was unnecessary, but were convinced that permission to use it allowed food manufacturers to be very careless in their methods of manufacture.
In the early days of enforcement many of us thought, Dr. Wiley, that you were too radical in your ideas of pure food and felt that you were doing harm to our industry. When I look back over the changes that have come to the food industry during the past twenty-five years and see the great changes for the better that have come to our methods and our products, I wonder why we were all so blindly asleep as we were and why, much sooner than we did, we did not welcome and follow your teaching.
A final point Wiley made about chronic illness and our food supply also holds just as true now as it did then:
The deplorable condition of our young men was vividly shown in the Great War [World War 1]. Fully one-third of those called to the colors were found to be physically and mentally unfit to serve their country in its hour of need. Another third could only attend to camp and hospital tasks. Only one-third could go into the trenches and serve their country on the field of battle.
It was a matter of supreme importance to endeavor in all honorable ways to remove the possibility of a similar stigma which might arise from any future crises of the republic. To instruct young persons to be parents, to teach them how to bring up their children after they are born, and to eliminate such a percentage of unfit are problems which require careful study.
Note: RFK has repeatedly emphasized that our nation faces a grave national security risk as a recent Pentagon study found 77% of young Americans are ineligible to serve in the military due to their pre-existing health issues (e.g., obesity).
The Kefauver–Harris Amendment
In the years that followed Wiley’s departure, the handicapping of the FDA continued and it drifted towards specificity (making sure it did not act in error) rather than sensitivity (taking bad things off the market) until thalidomide completely flipped things in the other direction.
Discovered
in 1952, thalidomide began being marketed in 1957 (initially over the
counter) as a miracle cure for morning sickness, insomnia, colds, and
headaches, and before long 14 pharmaceutical companies were selling it
in 46 countries under at least 37 trade names. Reports soon emerged of
infants born with partially missing limbs. In 1959, it was observed to
cause peripheral neuritis. At the end of 1961, it was taken off the
German market in November and then globally in December after an Australian doctor was finally able to get a letter published in the Lancet about it causing birth defects (after having unsuccessfully tried to sound the alarm since June of 1961).
Note:
during its brief availability in Germany, thalidomide was estimated to
have caused over 10,000 birth defects and the deaths of approximately
2,000 children.
Thalidomide’s adoption in
America was slower since the initial American company its manufacturer
approached found it lacked efficacy in their preliminary trials and
hence didn’t want to market it. By the time a second company began
testing it across America at the end of 1960, widespread concerns
existed about thalidomide. This led the FDA reviewer assigned to
thalidomide, Frances Oldham Kelsey, who was not allowed to block thalidomide from coming to market, to instead repeatedly stall its approval.
As a result, roughly 20,000 American women received it during the extended clinical trials (with many injuries being observed throughout that period by the FDA). Still, it was kept away from the general population (excluding doctors who gave it to their personal circle because the manufacturer had not told them it was still experimental). Kelsey’s actions in turn, resulted in only 17 American birth defects occurring (from the preliminary testing done across America) and earned her a presidential medal from John F. Kennedy on August 7, 1962.
Since the FDA had lacked sufficient authority to block toxic drugs from coming to market, the near miss with thalidomide got Congress to unanimously pass the 1962 Kefauver–Harris Amendment, a law that required drug manufacturers to prove their drugs were “safe and effective” before bringing them to market, and
While this law was necessary, it also was highly misguided as it allowed the Secretary of Health and Human Services to also block a drug if:
There is a lack of substantial evidence that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the proposed labeling thereof.
The term 'substantial evidence' means evidence consisting of adequate and well-controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the drug involved, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof."
All of this led to a few major problems.
First, Kelsey’s actions dramatically increased the prestige of the FDA, both emboldening the agency and simultaneously leading to many other jealous officials wishing to get the recognition she did for stopping the next thalidomide (which DMSO, discussed later in this article, conveniently fit the profile of).
Because of this, the pace of new drugs entering the market dramatically slowed, and ever since then, a consistent complaint of Congress has been the FDA blocking medical therapies the public needs. For example, in each Congressional hearing over the FDA stonewalling DMSO, the Senators and Congressmen always emphasized the importance of a proper balance between the FDA being able to block harmful drugs while approving good drugs, but stated the pendulum had swung too far towards specificity, as since the law had passed the number of new drugs entering the market had slowed to a standstill.
Secondly, it galvanized the FDA into rapidly establishing its authority and creating numerous divisions to “police” questionable drugs without the organization being structured to effectively or appropriately administer that authority (which led to perpetual mismanagement, chaos, and frequent abuse of that power). For example, James Goddard (the FDA commissioner from 1966-1968) believed the organization required police powers to keep the scientific community in line, and used a variety of chilling tactics to enact this precedent (e.g., conducting unannounced raids and defaming researchers in the national media). On the one hand, I can sympathize with this perspective because over the decades the FDA had lost so much of its “sensitivity” in keeping harmful drugs off the market, but at the same time, he swung the pendulum far too far, and left the FDA with such poor specificity that it began habitually engaging in unjustified enforcement actions that significantly harmed the American people (e.g., by keeping DMSO from the country).
Third, the law required efficacy to be a “well-controlled” trial. This became a massive problem as the FDA relentlessly chose to define “well-controlled” as a double-blind trial (to the point they clung to this specific argument in 1980 when Congress and the Senate grilled them over their decision to stonewall DMSO).
This was a huge issue because:
•I believe it enshrined the scientific supremacy of randomized controlled trials (RCTs).
•RCTs
are extremely expensive. As such, most can only be done by the
pharmaceutical industry, which due to their cost, consistently frames
them (presented in favorable ways, ignoring or adjusting harmful data)
to protect the company’s investment (which leads to RCTs frequently
being highly inaccurate). This in turn, rapidly increased the cost of
drug approval, effectively turning drug approval into a pay-to-play type
situation (e.g., currently, the cost to bring a new drug to market is
estimated to be between 0.98-4.54 billion dollars, which makes it impossible for any unpatentable product ever to get FDA approval).
•RCT
fundamentalism is highly misguided as smaller observational unblinded
trials will typically yield the same results as large (non-corrupt) RCTs
(proven by this 2014 Cochrane Review),
especially if the effect of a drug is significant (rather than a tiny
one that can only be detected in a large controlled study and hence is
likely inconsequential). This matters because most innovative therapies
are developed by clinical observations that lead to those smaller
trials, but due to RCT fundamentalism, all of that compelling research
is inevitably thrown in the trash. For example, anytime someone uses an
effective therapy that competes with the medical monopoly, if costly
RCTs have not been performed, the establishment always cries “there’s no
evidence” for the therapy. It uses this to justify stonewalling it and
targeting anyone who provides it.
Finally, once these provisions became enacted and the FDA began targeting “unapproved” therapies, the number of remarkable medical innovations produced in America dramatically declined. This decline in turn, paralleled the overall decline in disruptive science, which swept America as scientists faced more and more pressure (e.g., from the grant system they depended upon) never to investigate unorthodox ideas that challenged existing paradigms. As such, in recent decades, we’ve had surprisingly few major discoveries that rewrite the rules of science despite the technology that could make those discoveries having significantly advanced.
What’s particularly sad about this is that many of the forgotten medical therapies I use in practice were developed between the 1920s to 1960s, as this was the time when technology had advanced enough to begin making “cutting edge” medical therapies, while the censorship apparatus had not yet evolved enough to keep them from seeing the light of day.
The FDA in the 1970s
Because the FDA had rapidly expanded in numerous directions it was not prepared to do, it frequently failed to fulfill its primary responsibilities (e.g., taking something harmful off the market), and it simultaneously took things away Americans actually wanted. This in turn led to numerous committees investigating the FDA (e.g., Commissioner Lay’s Kinslow report of his agency’s serious shortcomings) and key officials with integrity like Lay being kicked out, all of which were encapsulated a series of scathing articles that were published in the New York Times in 1977 (e.g., this, this, this and this one), which included passages such as:
But the agency, a bureaucratic waif that is responsible for overseeing a staggering $200 billion worth of products yearly, is not only whipsawed by the public controversy, it is so demoralized that a number of its top positions long go unfilled, so burdened that it cannot keep up with the explosion of consumer goods and so battered by lawsuits and outside pressures that its power to make its decisions stick is sometimes undermined.
Its bureaucratic problems have been so vexing that in just the last three years the agency has been the target of more than 100 Congressional investigations, 50 highly critical reports by the General Accounting Office and a series of internal inquiries despairing of ever setting the place right.
The Congressional hearings in the last couple of years just about destroyed the agency,” an agency official said privately. “The staff has been torn by dissension and strife, the morale is bad, there's no direction and stagnation has set in.”
Indeed, after his departure as Commissioner of the agency in 1969, Dr. Herbert E. Ley said that “what the F.D.A. is doing and what the public thinks it's doing are as different as night and day.” He complained further that during his 18‐month tenure he had been under “constant, tremendous, sometimes unmerciful pressure” from drug industry officials.
As problems arise the agency becomes embroiled in thousands of cases, some of which develop into national controversies, and at times it seems that the agency lurches from crisis to crisis.
A year ago the Ford Administration was on the verge of releasing an economic‐report containing scathing criticism of the agency's utility and effectiveness. The comments were later deleted for unexplained reasons.
Key administrative positions at the agency have sometimes gone unfilled for years and as a result various departments have been allowed to drift and founder through lack of leadership and authority.
Groups of dissident employees have trooped to Capitol Hill to testify against their superiors, plunging the agency into name‐calling internal squabbles that remain unresolved.
The internal complaints have also concerned lower level employees, with some agency officials privately describing members of the F.D.A.'s professional staff as “retreads” and “has beens.” In testimony a year ago dealing with low morale at the agency, Dr. J. Richard Crout, director of the Bureau of Drugs, said this about the chaos in which he had found the agency:
“There was an enormous documents room . . . where some people said fights went on and there was absenteeism. There was open drunkenness by several employees, which went on for months. There was intimidation internally. I tell you that in my first year at F.D.A., even lasting longer than that, 1972‐73, going to certain kinds of meetings was an extraordinarily peculiar kind of exercise.
“People—I'm talking about division directors and their staffs—would engage in a kind of behavior that invited insubordination. People tittering in corners, throwing spitballs—I'm describing physicians. People who would, let me say, slouch down in a chair, not respond to questions, moan and groan with sweeping gestures, a kind of behavior I have not seen in any other institution as a grown man.”
In summing up hearings of the two subcommittees, Senator Kennedy said last summer: “During the past two years these subcommittees have received testimony from 30 F.D.A. employees about the practices and internal management of the agency.
“These accounts included serious allegations of undue industry influence, improper transfers, details or removals, alteration of files and forced withdrawal of memoranda, bias toward drug approvals, improper manipulative use of advisory committees, disappearance of critical agency action memoranda into what the F.D.A. Commissioner termed ‘a mysterious bottomless pit,’ and incredibly slow moving ineffective enforcement and compliance programs with years elapsing between the discovery of a problem and the initiation of a solution, and inappropriate use of medical officer recommendations.”
Such disputes wear and divide further an agency that in recent years has been accused in lawsuits of incompetence or wrongdoing, has been investigated more than 100 times by Congressional panels and has had its intent challenged by liberals and conservatives. All the while, new products continue to be spewed out by the score, while the agency says it cannot monitor those already on the market.
The 766‐page report of the group, headed by Norman Dorsen, a professor at the New York University Law Center, cited detailed cases of harassment of staff by F.D.A. officials, insubordinate behavior by professional staff and inordinate delays in making recommendations on the quality of new drugs.
In my eyes, the most important thing about this period of FDA reforms was that the FDA was the most complained about agency in the government, and Congress made numerous attempts to fix it (and ethical FDA officials). Still, nonetheless, the situation described in the NY Times series persisted throughout the agency.
The DMSO Saga
Over the last three months, I’ve begun exploring a remarkable forgotten side of medicine—DMSO, a simple and freely available natural chemical that is one of the safest substances in existence and incredibly effective at treating a variety of conditions, including many which are otherwise impossible to treat.
In turn, once DMSO discovered in the early 1960s, it spread across the country like wildfire as patients immediately saw it treat a variety of debilitating conditions (e.g., chronic pain or severe arthritis), and researchers realized that it represented a new therapeutic principle that would completely transform medicine. Before long, the entire research community had gotten behind it, as they realized DMSO’s remarkable properties would completely transform the practice of medicine. Likewise, since opening up this topic, I’ve received over a thousand reports from readers who have had almost unbelievable results from DMSO that precisely match what many reported in the 1960s and 1970s.
For reference, those conditions included:
•Strokes,
paralysis, a wide range of neurological disorders (e.g., Down Syndrome
and dementia) and many circulatory disorders (e.g., Raynaud’s, varicose
veins, hemorrhoids), which I discussed here.
•A wide range of tissue injuries such as sprains, concussions, burns, surgical incisions, spinal cord injuries (discussed here).
•Chronic pain (e.g., from a bad disc, bursitis, arthiritis or complex regional pain syndrome), which I discussed here.
•A
wide range of autoimmune, protein and contractile disorders such as
scleroderma, amyloidosis, and interstitial cystitis (discussed here).
•A variety of head conditions, such as tinnitus, vision loss, dental problems, and sinusitis (discussed here).
•A wide range of internal organ diseases such as pancreatitis, infertility, liver cirrhosis (discussed here).
•A wide range of cancers, infections, and skin conditions.
This all raises a simple question. How is it that no one knows about DMSO or that an agent which could dramatically reduce the need for opioids or prevent millions with stroke and spinal cord injury from having a life of disability never saw the light of day?
Quite simply, the FDA and the pharmaceutical industry were initially extremely interested in DMSO. However, once the FDA recognized the scale of new drug applications they would need to process, to get out of having to do that, they reversed their position and declared (without evidence) that DMSO was an extremely dangerous substance, and threatened both the pharmaceutical companies (who had already made significant investments to bring DMSO to market) and each researcher in the country who was giving it to patients in an unapproved manner.
Before long,
the head of the FDA (Goddard) realized demonizing DMSO (a “dangerous”
drug that was rapidly spreading across the country and being used by
“irresponsible” researchers in an “unapproved” fashion) would give the
FDA justification for the police powers he sought for the agency. In
turn, due to both Goddard’s crusade and the FDA (like any other
government agency) not wanting ever to admit they had been at fault, the
agency continued to double down on the claim “DMSO was dangerous” even
after an exhaustive safety study demonstrated that taking 10 times the typical DMSO dose for 90 days posed no risk to human participants.
Note:
one journalist who interviewed multiple successive FDA commissioners
and reviewed all the guidances the FDA put out about DMSO was struck by
how little the FDA’s leadership understood about the most controversial
drug in America and how misleading and inaccurate (and unreferenced)
the FDA’s DMSO guidelines were.
Since many people relied upon DMSO to treat their chronic pain (along with a variety of other debilitating injuries), physicians, patients, politicians, and celebrities resisted this encroachment, and after years eventually forced a Congressional and then a Senate hearing. In these hearings, the FDA was confronted with a mountain of evidence (and numerous compelling patient stories) showing DMSO did indeed work (including for numerous terminal conditions which desperately needed treatment). Nonetheless, the FDA continually stuck to the claim there was “no evidence” DMSO worked, as blinded placebo controlled trials had never been conducted with DMSO—something which had never been done because of DMSO’s unique properties (e.g., the temporary skin irritation, characteristic odor and rapid dramatic improvement it frequently causes) made it impossible ever to conduct a truly blinded trial on it.
At both hearings, the FDA promised the legislators that any future drug applications for DMSO would be given a fair chance. However, this never happened, and the embargo persisted for decades as the FDA continued to escalate its war against natural medicine. This eventually cumulated in a completely unjustified 1992 raid on a famous integrative medicine clinic (which Dr. Wright describes below).
As these events occurred prior to Clinton legalizing direct to consumer pharmaceutical advertising in 1997 (and hence allowing the pharmaceutical industry to buy out the news), a recording of it made the nightly news and quickly went viral across America. Soon after, in 1994, due to outrage over the FDA’s increasingly draconian war against natural medicine, the Dietary Supplement Health and Education Act (DSHEA) passed, a law that essentially took away the FDA’s ability to regulate naturally occurring supplements.
Since DMSO was a naturally occurring supplement, this law effectively legalized it, and it is now widely available. It is hence my sincere hope (which appears actually to be occurring now) is that it will be possible for the public to at last remember what was done with DMSO in the 1960s (when hundreds of thousands of Americans used DMSO), and in time this can create the pressure to bring it back to the hospitals, the place where it can most benefit patients (e.g., for strokes).
Note: I provided a much longer summary of the FDA’s war against DMSO here as it provided a template for what they’ve done to many other natural therapies.
The FDA’s War Against Natural Medicine
Shortly before the election, RFK Jr. gave what I considered to be one of the most important statements in the entire campaign:
This tweet touched upon the fact that for decades the FDA has done everything it can to remove effective natural therapies from the market, something which I believe (but cannot prove) originated from the AMA’s monopolistic tactics which allowed them to become the most powerful force in American medicine.
The AMA’s original playbook was to pressure pharmaceutical (or cigarette) companies to sponsor the AMA in return for the AMA’s seal of approval on their pharmaceutical.
In time, it evolved into the FDA targeting each viable natural therapy and then pressuring the inventors to sell their rights to the AMA in return for the AMA “proving” the therapy worked, or if they didn’t sell out, the AMA burying it. This for example, is what happened to ultraviolet blood irradiation and numerous remarkable cancer cures (some of which I discussed here and here).
As the years moved forward, the government worked in greater and greater coordination with the AMA. Before long the FDA was the primary enforcer of “quackery” the AMA disapproved of, which eventually evolved into the FDA’s war on natural health.
For example, in a previous article, I provided a wealth of evidence that demonstrates that restorative sleep is one of the most important things for health and the major issue with sleeping pills—that almost all of them are sedatives that disrupt the natural sleep cycle. However, what many do not know is that one naturally occurring compound (found in foods and the human body), is an incredibly effective sleep aid that enhances restorative sleep.
As such, γ-Hydroxybutyric acid (GHB) was life changing for many (especially the elderly, people in occupations that forced them to have irregular sleeping hours and those with chronic illnesses like fibromyalgia) and at the time it was available, we saw many results physicians now have forgotten are even possible. Unfortunately, the FDA decided they didn’t want a competing sleeping pill on the market and began illegally raiding and arresting those who were providing GHB.
Once courts began throwing those convictions out, the FDA then pivoted to creating a national hysteria about GHB being a deadly date rape drug (despite the evidence showing this was not true). Eventually, it was able to attribute GHB to two deadly date rapes (when in reality it was not responsible for either), and pass a national date rape drug law which made GHB a schedule I drug, thereby bypassing its protections as a natural supplement under DSHEA and gave the harshest penalties possible for possessing it.
Curiously however, the act also
made GHB be a schedule III drug when it was given for an FDA approved
use (narcolepsy), and presently costs between $60,000 to $100,000 a year
(which patients are willing to pay because of how profoundly it
benefits them). Activists in turn, have fought for decades to expand the
approved uses of prescribed GHB (something which I believe would
profoundly improve America’s health), but aside from one other rare
sleep disorder, the FDA has (predictably) refused to do so.
Note:
GHB also helped with many other issues (e.g., healing tissue and
building muscle mass—something typically utilized by bodybuilders but
also critically important for the elderly). Most recently, a clinical trial discovered GHB treats laryngeal dystonia (the condition affecting RFK Jr.’s voice).
Sadly, that’s not by any means the only example. For example, in RFK Jr’s tweet, he mentioned a few others (e.g., the nutraceuticals and vitamins the FDA has tried to get off the market since Goddard’s time). I will briefly discuss a few of them:
•One of the most effective forms of psychotherapy combines it with psychedelics, which unfortunately, given its association with illicit drug use is highly controversial (e.g., most psychedelics are schedule I drugs) and thus has required decades of work to make the FDA open to allowing clinical trials of it. When MDMA is used for psychedelic assisted psychotherapy, it is particularly helpful for veterans with PTSD (of whom roughly 20 commit suicide each day). Given how dire the situation many veterans are in from battlefield trauma (for which conventional medicine has nothing to offer), there has been an immense bipartisan push to conduct the research needed to make it available to veterans.
Sadly however, when an FDA panel met to review the (fairly profound) data that had been gathered, they voted 9-2
against approving it, stating more evidence was needed to ascertain
that the benefits of the therapy outweighed its risk, largely because
the trials weren’t sufficiently blinded and hence not “well controlled”
(essentially the same situation DMSO found itself in as you can’t really
conduct blinded psychedelic sessions). As such, this decision was
attacked by both the left and right, and American veterans often have to
go to Mexico to receive this life saving treatment, something which has
sadly been a recurring theme with many of the essential therapies the
FDA prohibits.
Note: many of the other benefits of psychedelic assisted psychotherapy are discussed here and will be the focus of a future article.
•When milk is pasteurized, it denatures the proteins there, destroying many of the essential nutrients present, transforms milk into an allergen, and changes its electrical charge, making it go from a zeta potential enhancing substance to one that impairs it (e.g., causing congestion throughout the body). As such, many believe strongly in only consuming unpasteurized (raw) milk. This position is somewhat controversial because many bacterial illnesses have been traced to unpasteurized milk. However, in most cases, this is due to the milk being filthy in the first place, in essence making pasteurization only necessary for adulterated milk (which sadly characterizes much of the conventional food supply). Because of this, the FDA always tries to shut down anyone selling raw milk, but regardless of what they do, the demand for it persists.
•A good case can be made that sunlight is one of the most essential nutrients for life. Unfortunately, since it does not have a lobby to defend it, it’s often a convenient scapegoat for the ill health of our society. The dermatology profession monetized this by associating sunlight exposure with skin cancer, a highly misleading sleight of hand because the common skin cancer (which is never deadly) is associated with sunlight exposure, but the rarer (and deadly) form of skin cancer is actually due to a lack of sunlight exposure (as are many other cancers). Because of this, we are taught to be terrified of the sun giving us a deadly skin cancer, when in reality, it not only doesn’t, but avoiding it dramatically increases our risk of dying (e.g., a large study found smokers who get regular sunlight live longer than non-smokers who avoid the sun).
•Chelation therapy (especially when done at a low EDTA dose and without aluminum preservatives) is an incredible therapy that has been used for decades to restore health, particularly within the cardiovascular system (as it both decalcifies the arteries and, when dosed appropriately, restores the physiologic zeta potential). Sadly, the FDA has continually targeted physicians who practiced it for decades (however in 1978 and 1981, courts ruled against the FDA prohibiting doctors from conducting this practice) and still is antagonistic towards it (despite a large NIH study that was somewhat set up to fail nonetheless demonstrating EDTA helped cardiovascular disease).
•When used appropriately (which often requires avoiding higher doses), peptide therapy is remarkably effective for a variety of ailments. Unfortunately, the FDA has also been antagonistic towards these supplements, and this accelerated during the Biden presidency (e.g., it’s no longer possible to get some of the most important injectable ones).
•Umbilical cord blood stem cells (obtained from placentas that would otherwise be thrown away) are another truly remarkable therapy, and when used correctly, can produce life-changing results. Unfortunately, during the Biden administration, the FDA changed its regulations on cord blood stem cells and effectively made it impossible for the “unproven” product to be sold within the country, causing the companies that had built up an infrastructure to produce them to have to shut down (as there was no feasible way to get the FDA approval necessary to sell them).
Note: many other dysfunctional regulations also need to be addressed. For example, since the FDA will target anyone who sells infant formula made without seed oils, it’s impossible to find seed-oil free infant formula.
This arose from the Infant Formula Act of 1980 (which was virtually unanimously passed by Congress in response to more than 100 infants becoming seriously ill from nutritionally inadequate soybean oil-based formulas), as it contained outdated science from the 1970s (specifically these 1976 AAP recommendations which did not exist in the AAP’s 1967 recommendations), that required infant formulas to have at least 2.7% of its calories (300mg per 100 Kcal) comes from linoleic acid (the problematic fat in seed oils).
Beyond this making it illegal to sell infant formula without them, I and many others believe this is a root cause of the childhood obesity epidemic in America as seed oils impair mitochondrial metabolism and cause you to gain weight (e.g., this systematic review shows infant formulas cause excessive and rapid weight gain). As the law was written, the Secretary of Health and Human Services had the latitude to revise those outdated nutritional guidelines (but essentially never has).
Vaccine Coverups
Many have been horrified to learn that the FDA and CDC systematically ignored every possible sign the COVID vaccines were dangerous as they pushed it on more and more people (e.g., in this leaked recording, consider how stubbornly the head of FDA’s vaccine division refuses to acknowledge any of the evidence brought forward by a group of permanently injured vaccine recipients).
This severe betrayal of trust from our authorities thus made many ask, “How could this have happened?” In truth, this did not come out of nowhere. Rather it was simply the subsequent escalation of a longstanding tendency by the government to push vaccines they knew were unsafe and ineffective to market, synopsized in this remarkable presentation by Suzanne Humphries, MD, and covered in much more detail in this article:
In Humphries talk, she briefly mentions the following:
•Throughout
the history of vaccination, many vaccine disasters have occurred from
hot lots being released that injured or killed large numbers of people
(particularly in the military). While most of these were swept under the
rug, at the end of his career, one eminent bacteriologist compiled all
the instances he could get records of (which I detailed here).
Note: in my opinion, the evidence I compiled
demonstrates that hot lots (adulterated vaccines) are such a frequent
consequence of vaccination, it is virtually inevitable hot lots will
keep on hitting the market (e.g., we now have proof
this was a vital issue with COVID vaccine toxicity). As such, I believe
the only viable option is to have independent third-party testing (with
a robust system of audits for the testers so they can’t get co-opted by
industry) of all vaccines. This is the standard that many other
industries are held to (e.g., many products made by compounding
pharmacies), so there should be no reason why it can’t also be applied
to vaccines.
•When developing the polio vaccine,
a key challenge Salk faced was administering enough formaldehyde to the
polio virus to inactivate the virus but not enough that it deformed it
to the point it could no longer solicit a sufficient antibody response.
Due to the national fear surrounding polio, an “emergency” existed which
justified an expedited 1955 approval and Salk advocating for an
(untested) accelerated manufacturing process which was at risk of
containing live polio viruses. Then, two weeks after the vaccine was
released, cases began emerging across America of children who had become
paralyzed in the limb that was injected with Salk’s polio vaccine.
Note:
to produce the emergency COVID vaccines at scale, a novel manufacturing
process was used, which caused them to be contaminated with dangerous DNA-altering bacterial plasmids.
In the subsequent investigation, it was discovered that many of the labs that produced the polio vaccines had never had their product tested on humans, and an NIH researcher (Bernice Eddy) had already discovered that those vaccines caused polio. Still, her bosses decided to release the vaccine nonetheless.
•Five years later (in 1960), Eddy also discovered that the polio vaccines were contaminated with a cancer causing virus (SV-40). Her superiors (who’d already discovered it the previous year) decided not to disclose her findings to the public (to maintain public trust in the vaccine program). Eddy then bravely presented them at a cancer conference, after which she was demoted and lost her lab. It was not until 1963 (as evidence of the problem continued to mount) that the federal government forced the vaccine manufacturers to stop growing the vaccine on SV-40 contaminated monkey kidneys—at which point between 40-98 million Americans (and many more globally) were infected—although a case can be made SV-40 was present until around the year 2000 in some of the vaccines (e.g., we still frequently find it is critical to treat the SV-40 component of cancer).
This caused a massive cancer wave, which until the even worse COVID-19 vaccines, was completely unprecedented in American history.
•When the earliest influenza vaccines hit the market in 1945, Joseph Anthony Morris PhD was recruited by the Division of Biologics Standards (DBS) to assess vaccine safety and efficacy, eventually becoming the Chief Vaccine Control Officer. Morris discovered the influenza vaccines were unsafe and only worked between 0-40% of the time, and had safety issues. Still, his superiors ignored his data and released the “safe and effective” flu shots.
Like Eddy, Morris also faced significant retaliation, being harassed, demoted, losing access to his lab, and blocked from publishing his results. Before being fired, Morris decided to fight the FDA’s gross misconduct by hiring a lawyer and going to the Senate. This prompted a 1972 Senate hearing, which concluded that the issues Morris raised were only the tip of the iceberg. As a result of the hearing, thirty-two unsafe and unproven vaccines were taken off the market. Most of the DBS’s conduct was deemed so egregious that it was scrapped and replaced with a division in the FDA (which unfortunately did not fix the rot within the agency).
After being transferred to the new FDA agency, Morris continued to be responsible for overseeing our influenza vaccines. Then, in February of 1976, a swine flu strain was found in a soldier who died in March, leading to the government trying to drum up as much fear as possible about an apocalyptic repeat of the 1918 influenza. Morris was called to investigate and (correctly) proved this swine flu posed no risk whatsoever to America.
Since that swine flu strain reproduced slowly, it was not feasible to produce enough of it to make a vaccine before the “pandemic” faded into memory, so they hybridized it with the fast-growing 1918 influenza. Morris thought this was insane, but was again gagged by his superiors, so he went on a national speaking tour and was given a platform by sympathetic television hosts (as the pharmaceutical industry had not yet bought out the media).
Sadly, Morris was
not listened to, and the experimental vaccine was distributed across
America. Before long, the injuries piled up, with hundreds becoming
paralyzed from Guillain–Barré syndrome, dozens dying, and thousands of
lawsuits being filed.
Note: given that I and colleagues
knew many people who were severely injured by this vaccine, I am
relatively certain the official figures I just cited significantly
understated the actual number of injuries.
However, the one bright side to this was it occurred in an era when 60 Minutes was willing to do a segment on the disaster (which has numerous remarkable parallels to what happened during COVID-19).
Many more disasters were also green-lighted by the FDA. For example:
•During the Gulf War, a faction within the Department of Defense (DoD) which was deeply invested in bioweapons research, convinced the military to give American soldiers a variety of agents to protect American troops from Saddam Hussein’s suspected biological and chemical weapons. Since, many of the agents they used were experimental, the DoD convinced the FDA to author an “Interim Rule,” which gave the FDA commissioner the authority to override all the protections soldiers had against receiving experimental drugs (e.g., informed consent) if the commissioner deemed doing so necessary for a military objective.
Unfortunately, the military failed to follow the procedures the FDA had laid out to test experimental agents on soldiers. This ended up being a massive issue because approximately 250,000 of the 697,000 U.S. veterans developed a severe chronic illness known as Gulf War Syndrome that was tied to these experimental agents, particularly the anthrax vaccine (whereas in contrast, Hussein never deployed biological or chemical weapons on US troops). Remarkably, rather than pull the vaccine, the military continued to mandate it on our troops, and eventually in 1998, the FDA forced the manufacturer to temporarily stop production, due to their rushed “emergency” vaccines being heavily adulterated and much different from had been promised (and likely why it injured so many people).
Note: the devastation of the anthrax vaccines is hard to put into words and in many ways, served as a blueprint for the cruel military COVID-19 mandates. I covered it in more detail here.
•In
the lead up to the second Iraq war, George Bush tried to create a
national hysteria about Hussein releasing smallpox on America (which he never had). He began a mandatory vaccine campaign within the military
(and a voluntary one for first responders), which was then stopped
midway (after 578,286 military personnel and 39,353 healthcare workers
had been vaccinated) due to it causing myocarditis.
Note: those events (and the media coverage) are detailed further here.
As best as I can tell, the FDA enabled this one to happen, but was not
as belligerent in protecting the vaccine as it had been in the other
incidents.
•To make a lot of money, Merck pushed a new vaccine onto the market which could “prevent” cervical cancer, and then did a massive marketing campaign in coordination with the CDC and FDA to scare people senseless about cervical cancer (a relatively rare cancer). During the vaccine trials (in healthy young girls), an extraordinarily high rate of (often permanent) injuries happened (e.g., at least 2.3% developed permanent autoimmune disorders and 0.085% died). To hide this, Merck gave a harmful “placebo” (the autoimmune triggering vaccine adjuvant) to the control group, so the high rate of injuries in the vaccine group would be comparable to the “placebo” and thus not linkable to the vaccine.
Once
the HPV vaccine hit the market, the FDA was immediately deluged with
reports of severe vaccine injuries (on a scale that had never been seen
with any previous vaccine). Still, the FDA and CDC chose to do
everything they could to protect (and promote) the vaccine regardless of
the public protest it received. Much later, it was also discovered the
trial data showed the vaccine increased your risk of developing cervical cancer if you were already vaccinated. Twenty years later, there’s very little evidence the vaccine reduced cervical cancer at all (which it was never actually proven to do) and quite possibly increased it.
Note: the parallels between what happened with the HPV and COVID vaccine disasters are extraordinary, and I covered them here.
More Coverups
Sadly, the FDA’s tendency to relentlessly protect a drug it approves is not unique to vaccines (e.g., consider Dr. Graham’s testimony about Vioxx at the start of the article). Of those scandals, I believe the SSRI antidepressant saga (detailed here) provides the closest template of what happened with the COVID vaccines.
When the SSRIs were originally developed, the evidence for them was so poor, it took (admitted) bribery to get the first one, Prozac, approved. Since the Bush family had close ties to Prozac’s manufacturer, once it got an overseas approval, George HW Bush pushed it through here. Unfortunately, the SSRIs, beyond having very poor evidence they helped depression, had a variety of frequent severe side effects, including frequently emotionally anesthetizing the recipients, 20-40% of users developing Bipolar syndrome, 59% getting sexual dysfunction (which makes you depressed), and a 255% increase in suicidal tendencies. Worse still, they had a tendency to cause violent and psychotic behavior (e.g., mass shootings, grisly homicides, and most commonly violent suicides), frequently causing the user to behave in a manner sometimes analogized to “being possessed.”
Because of this, once the SSRIs came out, the extraordinary personality changes they created caused them to rapidly become the most complained about drug in America. Remarkably however, the FDA refused to listen to the public, and even gagged their own scientist from publishing a report showing they caused children to commit suicide. Much later, lawsuits revealed all these issues were discovered throughout the clinical trials, but the FDA hid it from the public, and to this day still relentlessly defends the drugs.
Many other controversial approvals can be cited as well. Of these, the most recent one happened with a new Alzheimer’s drugs that eliminate amyloid plaque from the brain (something which few know the brain actually produces to protect itself from damage). The first drug failed to show any improvement for Alzheimer's disease, while brain swelling or brain bleeding was found in 41% of patients enrolled in its studies, resulting in the FDA’s advisory panel (which typically votes yes) voting 10-1 not to approve it.
Nonetheless, the FDA overrode their panel, leading to three resigning from the panel. Congress then investigated the approval (because the drug was so expensive it had the potential to bankrupt Medicare) and discovered numerous red flags (e.g., the FDA sidelined its scientists who raised concerns about it, and the FDA helped the manufacturer prepare its presentation to the FDA advisory committee). Despite all this, the FDA kept it on the market, but fortunately, it was so unsafe and ineffective, that doctors were unwilling to use it, and before long the manufacturer was forced to pull it from the market.
Shortly after, another Alzheimer’s drug was brought to the FDA, and this time, the commissioner decided to bypass the advisory panel and instead gave it a backdoor approval. Immediately after, he was a keynote speakerat an annual pharmaceutical investment conference where JP Morgan hyped up the incredible profits both the new Alzheimer’s and Obesity drugs (e.g., Ozempic) would offer in the years to come. As you might expect, when each of those Alzheimer’s drugs was approved, the agency released a glowing press release and since then has pushed Ozempic on America’s children.
Fixing the FDA
At this point, I’ve seen a variety of proposals put forward to fix the FDA, which alternate between reforming the agency (e.g., Robert Malone wrote one of the best summaries of what needs to be done in this regard) and scrapping the agency entirely.
In my eyes, the core dilemmas are:
•The
FDA performs a variety of necessary functions to protect the public, so
eliminating it entirely would be a disaster. Likewise, if it were
scrapped (like the DBS was in 1972), whatever followed it would likely
be just as bad if not worse because too many of the bad parts would
remain.
•Because corruption has been so systemic
within the FDA, even when someone who genuinely wants to reform the
agency takes charge, their efforts go nowhere, and are quickly
overridden by a corrupt successor. As such, the majority of reforms
proposed are likely only to create a fleeting impact.
•The
task the FDA has to do is so immense it’s simply impossible for them to
get everything done. Because of this, they regularly outsource
evaluating safety and efficacy to pharmaceutical companies (which
essentially defeats the point of having the FDA exist to protect the
public) and make drug approval a pay-to-play situation where approval is
based on how much money you put in, not the quality of your product.
•Like
many government agencies, the FDA never likes to admit it was wrong, so
once a drug is approved (e.g., by the right people being bribed),
regardless of what the public does or what data emerges, it will almost
never revoke that approval (e.g., consider what we’ve seen throughout
COVID-19).
•Since the FDA’s resources are limited,
it will always default to attacking natural medicine, as unlike
pharmaceutical companies, natural medicine providers can’t fight back,
thereby allowing the FDA to create the facade of protecting the public
without having to fear lobbyists getting the government to retaliate
against the FDA. In essence, all the agency's issues can be boiled down
to “selective prosecution.”
Note: similarly, the natural
health industry can’t bribe FDA employees with future high paying jobs,
whereas the pharmaceutical industry routinely does that.
In my eyes, the best solution to this problem is hence to restructure the FDA (and the public’s relation to it) so that the systemic corruption within the agency (which is almost inevitable given how much money lies in medicine) does not prevent it from serving the public interest. Fortunately, there are quite a few ways to do this. A few of the ideas I’ve looked at include:
1. Have a law in place that voids the vote of anyone in a healthcare related committee or panel who is later shown to have (or to develop) financial conflicts of interest with the relevant pharmaceutical company, which then reverses decisions that would no longer pass once the corrupt vote was nullified, and similarly to apply the same standard to individual regulators who push drugs through. If this is in place, it makes it much harder to bribe healthcare decision makers (as one of the most common forms of bribery is providing a lucrative job or grant to them after they help the company—e.g., the FDA commissioners who pushed the COVID vaccines all subsequently got lavish jobs with their manufacturers).
2.
While I believe it’s essential for the FDA to evaluate safety and
ensure products contain what they claim, virtually everyone I’ve spoken
to believes the FDA should not be responsible for assessing efficacy.
This is because “efficacy” is an incredibly subjective metric, and time
and time again, you will see the FDA approve a drug for an
inconsequential “benefit” (which was likely the result of statistical
manipulation of the trial data or outright fraud), but simultaneously
refuse to approve a drug with a wealth of evidence behind it. As such,
two parallel approval tracks needed to be created:
•One that follows the existing framework and makes medications eligible for insurance reimbursement.
•One for alternative therapies that have a longstanding record of safety (e.g., ultraviolet blood irradiation
has been in regular use for almost 100 years and demonstrated a high
degree of safety throughout that period) but with divided opinions on
their efficacy. Therapies in this category would not require FDA
“approval” to be used (or studied in community trials), provided their
safety is superior to the existing therapeutic options.
Note: many have recognized the need for this alternative track over the decades (e.g., throughout the AIDS crisis,
physicians [and legislators] across America fought to get FDA approval
for community trials for therapies that were saving AIDS patients), and the Right To Try Act
passed during Trump’s first term attempted to do this. Unfortunately,
the terms of that act were too restrictive. For example, when we tried
to use that law during COVID, we found it still was not possible to use
“experimental” therapies (with supportive data but not an FDA approval)
on patients who were otherwise expected to die (and then typically did).
3.
Currently, our entire system judges efficacy by the FDA’s seal of
approval, when in reality that seal is often highly misleading. As such,
I (and many others) believe the marketplace is actually a better metric
for determining efficacy, as consumers will typically only pay for
things they have a strong basis to believe work (whereas many of the
costly therapies patients receive they would never purchase were
insurance not covering them).
Note: this policy would also require the justice system, medical boards, and malpractice lawsuits to recognize the validity of the second category (whereas since they currently don’t, that legal liability is one of the primary reasons why many doctors will not use “unapproved” therapies).
4. The evaluation of drugs and pharmaceuticals needs to be done in collaboration with the public. Presently, there is simply too much data for the drug regulators to be able to go through, so without members of the public (or AI systems the public has access to) evaluating the data pool, critical red flags will be missed and not be acted upon. For example, during COVID, a community of independent scientific journalists who, with minimal data, were able to rapidly identify a wide range of safety signals, regulators around the world had missed. Given that COVID was the primary focus of the regulatory agencies, this suggests that many other critical items are being overlooked and that outside observers are required to identify them.
5. Similarly, during the drug approval process, the data that led to drug approval needs to be available for the public to examine (rather than only being attainable through a drawn-out legal process). If this had been done, the systemic fraud throughout the COVID trials and the lack of safety or efficacy from the vaccines would have been immediately exposed (rather than the FDA ignoring those reports and instead reporting the whistleblowers to Pfizer). Sadly, this issue is by no means unique to the COVID vaccines (the actual data on many approved drugs is atrocious), and were it known, many would immediately be pulled from the market.
6. There need to be ways to revoke a drug approval if the FDA continues to protect a lousy drug, which as things like Vioxx, the SSRIs and many catastrophic vaccines show, it always does regardless of how strong the data is against the pharmaceutical or how much public protest it meets. Remarkably, at this point, it can’t even be done at a state level (as much in the same way the Supreme Court prohibited states from banning toxic food additives, courts have found FDA approvals supersede state laws). The only remedy is a protracted court process, which if lucky can force the FDA to require a warning label on the drug.
Exactly
how this provision would be implemented is less clear, as if it’s
simply shunted to another agency, lobbyists can still overturn any
attempts to prohibit it. As present, the best solutions I’ve come up
with are to:
•Lower the bar required to successfully sue
the manufacturer of an FDA approved drug or the prescribing physician
if the effect was not adequately disclosed or monitored (as if doctors
did that, patients would not want to take the more toxic drugs, and
doctors would prescribe far less of them).
•Restore a state’s right to ban drugs.
•Give courts the legal authority to pull dangerous drugs.
•Have
a provision in place where if a sufficient number of individuals sign a
petition to have a drug’s safety re-evaluated, a committee is randomly
selected from a large pool of applicants that can evaluate the existing
evidence from both sides and then override the FDA’s approval if
significant safety concerns exist.
Note: the intention
behind pulling from such a large pool of potential experts is to make it
impossible to buy out the committee (which typically is what always
happens).
Conclusion
In my eyes, the primary thing which characterizes the modern era is longstanding paradigms rapidly changing (think how different the world has become each decade compared to how slowly things changed a few hundred years ago). One of the most tremendous forces accelerating this process has been social media (particularly Twitter) breaking the stranglehold on truth the legacy media once had.
For almost a century, public policy has been decided by narratives being disseminated in unison by all the major media outlets, but now that they no longer have a monopoly on information, counter narratives can quickly go viral, and if they have a strong validity behind them, rapidly outcompete the mass media’s propaganda. As such, there is now much more pressure on authorities to be truthful, and as blatant lies are becoming much easier to expose. Similarly, the primary reason groups like the FDA or the CDC have had so much power has arisen from so many other organizations deferring to their guidance, and the media in lockstep supporting it.
Ultimately, I believe that if we want to Make America Healthy Again, the solution will not be to try to “fix” the government but rather use the limited window we have now to rapidly accelerate the public’s ability to become directly involved in healthcare policy, as that way, even if the government changes, the public will still be able to counteract its bureaucratic encroachment effectively.
For example, there has been a longstanding embargo on obtaining any medical data which threatens government or corporate interests. A key point RFK Jr. has campaigned on is making the CDC’s Vaccine Safety Database (compiled from a large number of electronic health care records) available to the public, as this would likely immediately give rapid and incontestable proof vaccines aren’t “safe and effective.” Likewise, I know existing artificial intelligence could do the same for many other pharmaceuticals if they had access to the EHRs. Immediately, both flag the most dangerous drugs but also tell us precisely how much they actually harm or benefit patients (which in turn would lead to many bad drugs rapidly being taken off the market).
Likewise,
there has also been a longstanding embargo on testing any integrative
therapy that performs significantly better than the existing standard of
care (e.g., we’ve tried numerous times, but the FDA makes it almost
impossible to conduct those trials—so it is again possible to argue
there is “no evidence”). As such, when I learned RFK Jr. was going to
announce his candidacy, I did a variety of things to help promote it. Then I shifted my focus to trying to make the case for the integrative therapies I believed would be the most likely to show a rapid benefit for hospitalized patients (e.g., ultraviolet blood irradiation and DMSO).
This
was done under the logic that if a clear and substantial benefit could
be proven for the therapies (which could be done the fastest for
hospitalized patients), it would be impossible to put the cat back into
the bag as the public would quickly start demanding those therapies in
the hospitals. At that point, they could diffuse into general use
outside of a hospital setting.
Note: through my network, I know that there are numerous hospitals and physicians who want to conduct those trials, so if the FDA’s barriers to them were removed and hospitals were rewarded by Medicare rather than penalized for doing so, they could get off the ground quite quickly. Likewise, I know there is considerable interest in community based trials of these therapies, provided the red tape is removed.
Most importantly, all of these incredible possibilities are only possible because of the work all of you have done to fight back against the COVID cartel and help shift society towards embracing a model of healthcare that puts people before profits. On the one hand, I am incredibly grateful to all of you for helping to bring this to pass (and spent much of Thanksgiving thinking about that), but on the other hand, it also means the next four years will be the time when each of us needs to become actively involved in improving America’s health, as that’s what we genuinely need to change things now.
To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here.




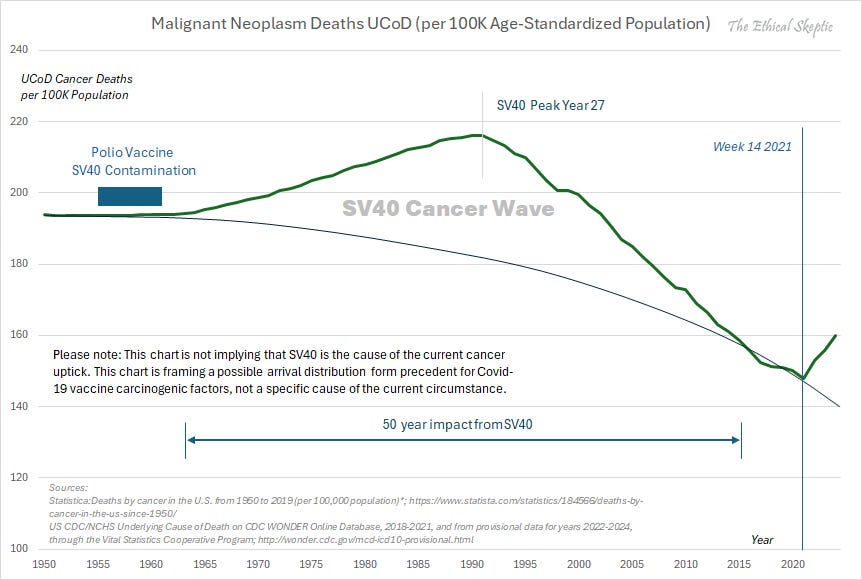
No comments:
Post a Comment