“To Evaluate the Safety, Tolerability, and Immunogenicity of BNT162b2 Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older” – Latest version (submitted July 14, 2023) on ClinicalTrials.gov
“The Safety of BNT162b2 Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older,” eh? Hmm. I was under the impression that if you are pregnant, or hoping to be, you shouldn’t do certain things like drink alcohol, smoke cigarettes, and get injected with experimental transfecting agents. You can find these updated clinical trial results here.

Just some notes about what they report here in this update with regard to serious adverse events (SAEs).
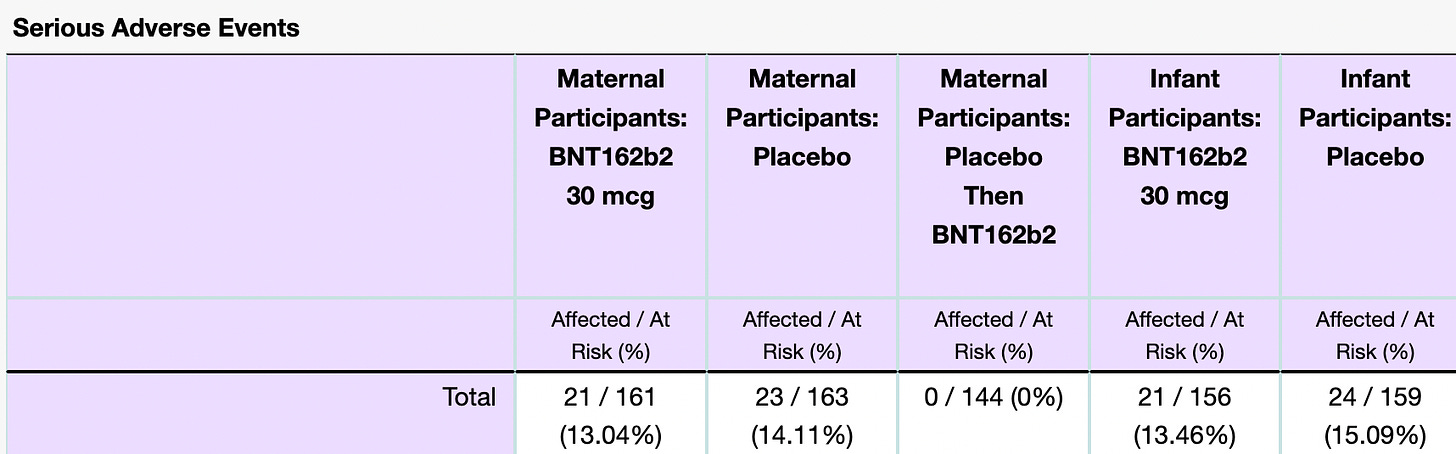
Perhaps the weirdest thing shown here are the percentages of SAEs when comparing the drug BNT162b2 and placebo. Here, once again, is how an SAE is defined in the context of this trial:

They looked at Maternal and Infant groups and included a total of 468 and 315 participants, respectively. Note: these are small numbers. Let’s look at Maternal cases first. In the cases of ‘Maternal Participants: BNT162b2 – 30 mcg’, ‘Maternal Participants: Placebo’ and ‘Maternal Participants: Placebo Then BNT162b2’ there were 161, 163 and 144 participants and in each group they reported 13.04%, 14.11% and 0% SAE occurrences. (I might refer to the BNT162b2 product as the drug.)
Some questions I have for the Sponsors/Collaborators of this trial:
- How did a higher percentage (1.07%) of women experience an SAE on placebo than on the drug?
- How did 0% of the women on placebo who subsequently got injected with the Pfizer product experience an SAE if 14.11% experienced an SAE on placebo alone?
- Is it not alarming in the first place that 13.04% and 13.46% of the participants who took the drug experienced an SAE? Not to mention that 14.11% and 15.09% experienced an SAE on placebo.
Answers?
- Perhaps, as many of us have been hypothesizing, the placebo is in fact, not a placebo at all. But they state very clearly in the ‘Study Description’ that the placebo that they used in this trial was saline.

For the people who do not know, saline (https://www.rxlist.com/normal-saline-drug.htm) is used as an injectable solution alongside drug injections in clinical trials as placebos based on the fact that saline does not induce an immunological reaction – i.e. an inert substance. (Rid A, Saxena A, Baqui AH, Bhan A, Bines J, Bouesseau MC, Caplan A, Colgrove J, Dhai A, Gomez-Diaz R, Green SK, Kang G, Lagos R, Loh P, London AJ, Mulholland K, Neels P, Pitisuttithum P, Sarr SC, Selgelid M, Sheehan M, Smith PG. Placebo use in vaccine trials: recommendations of a WHO expert panel. Vaccine. 2014 Aug 20;32(37):4708-12. doi: 10.1016/j.vaccine.2014.04.022. Epub 2014 Apr 25. PMID: 24768580; PMCID: PMC4157320.) It is meant to be used as a comparison – a control.
I am not going to get into all that now (Siri already revealed all we need to know via Offit already), but it is safe to say that saline would at the very most induce injection site swelling, redness, or infection, according to rxlist. I would imagine the reasons for these occurrences would be potential damage to the injection site upon needle puncture. So these would actually be things that might be usable as a placebo control in terms of ‘side effects’. The potential induction of fever is a bit weird to me. I would question this as well with the rxlist people. It might apply only to higher doses of saline or higher concentrations. I am not sure.
There also is a warning attached to injecting saline into people with ‘congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.’ But again, no SAE warning in sight for healthy people. And this makes sense, especially since it is considered to be an inert substance. What we are seeing here in this clinical trial in the placebo group in this clinical trial does not reflect an inert substance.
Here’s what I think is going on. And this is going to sound really awful. And I hope I am wrong. I think they published these results to enable using language like:
“The BNT162b2 product solicited similar rates of serious adverse events as for the placebo in Phase III clinical trials.”
in the future, in order to justify maintenance of the injection roll-out. Time will tell. Or perhaps it already is telling.
Not only safe, but beneficial. I see.
So how did a higher percentage of women experience an SAE on placebo than on the drug? Simple. They didn’t use saline. It’s the only logical explanation. If they used empty LNPs then this is incredibly telling with regard to the induction of SAEs by the LNPs themselves. In this case, it would appear in these small groups of women with short follow-up times that the LNPs containing modified mRNAs induce the same amount of SAEs than empty LNPs.
- How did 0% of the women on placebo who subsequently got injected with the Pfizer product experience an SAE if 14.11% experienced an SAE on placebo alone?
This one boggles my mind. I have no answer for this. All I can think is that it has something to do with timing of measurements. I welcome ideas here.
- Is it not alarming in the first place that 13.04% of the participants who took the drug experienced an SAE?
Yes. It is. The VAERS handbook states that 10-15% of reported AEs are classified as severe for any given set of data. (https://vaers.hhs.gov/docs/VAERSDataUseGuide_November2020.pdf)
But may I remind everyone here that this is not post-market monitoring pharmacovigilance of SAEs – this is a clinical trial with very small cohorts, and those percentages are toward the upper end of the highest classified percentages.
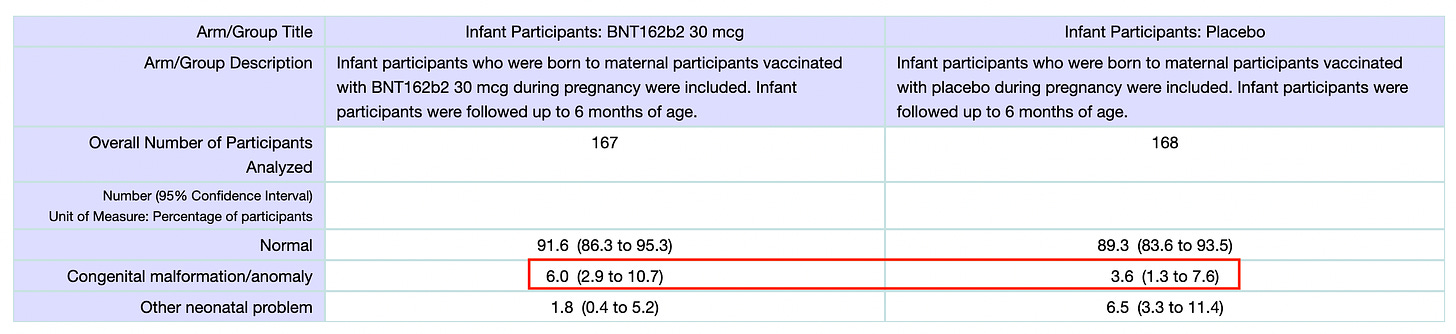
Now let’s look at infant cases. In the cases of ‘Infant Participants: BNT162b2 – 30 mcg’ and ‘Infant Participants: Placebo’ there were 156 and 159 participants and in each group they reported 13.46% and 15.09% SAE occurrences.
First off, same questions as for the moms. In this case, 1.63% more infants were reported to succumb to an SAE in the context of placebo! This makes no sense if the placebo was saline.
Also note that we surpassed the VAERS guidebook highest percentage here with regard to the SAE occurrences for infants on placebo (15.09%).
One more thing I would like to call attention to here is the fact that 1.7 times more infants were reported to have birth defects in the context of the drug. Now I don’t know how much water this result holds considering that we don’t seem to know what the placebo used was. But still, if we consider that they did use a saline placebo and it was shown to be less-associated with birth defects than the drug, then Houston, we might have a problem.
The reason we do these clinical trials is to test for safety, among other things, but let’s face it: who the hell is going to care if a drug works if it’s not safe? Depending on standards and guidelines, these trials may fail ‘the test’. So I have to ask: What are the standards and what are the guidelines here because, it seems to me that if the point of this particular trial was to find out if the drug was inducing serious harms to moms and infants then we did find out: it does. So, why do they call this a safe product?
Can we let the moms decide for themselves please? Let them know that the placebo is a big question mark, and that the drug induced 1.7 times more birth defects than the ‘placebo’. Let then know that the infants suffered more serious adverse events than what is considered acceptable – and this was in the context of placebo!
LET THEM KNOW.
This article was originally posted on Dr. Rose’s Substack.
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.


No comments:
Post a Comment