17,000+ Deaths Reported After COVID Vaccines, Including New Report of 12-Year-Old Who Died After Pfizer Vaccine
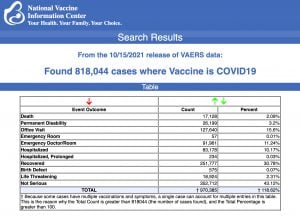
VAERS data released Friday by the CDC included a total of 818,044 reports of adverse events from all age groups following COVID vaccines, including 17,128 deaths and 122,833 serious injuries between Dec. 14, 2020, and Oct. 15, 2021.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
Data released Friday by the Centers for Disease Control and Prevention (CDC) showed that between Dec. 14, 2020, and Oct. 15, 2021, a total of 818,044 adverse events following COVID vaccines were reported to the Vaccine Adverse Event Reporting System (VAERS).
The data included a total of 17,128 reports of deaths — an increase of 362 over the previous week, and a new report of a 12-year-old who died after getting the Pfizer vaccine.
There were 117,399 reports of serious injuries, including deaths, during the same time period — up 5,434 compared with the previous week.
Excluding “foreign reports” to VAERS, 612,125 adverse events, including 7,848 deaths and 50,225 serious injuries, were reported in the U.S. between Dec. 14, 2020, and Oct. 15, 2021.
Of the 7,848 U.S. deaths reported as of Oct. 15, 11% occurred within 24 hours of vaccination, 15% occurred within 48 hours of vaccination and 28% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 406.1 million COVID vaccine doses had been administered as of Oct. 15. This includes: 237 million doses of Pfizer, 154 million doses of Moderna and 15 million doses of Johnson & Johnson (J&J).
The data come directly from reports submitted to VAERS, the primary government-funded system for reporting adverse vaccine reactions in the U.S.
Every Friday, VAERS
makes public all vaccine injury reports received as of a specified
date, usually about a week prior to the release date. Reports submitted
to VAERS require further investigation before a causal relationship can
be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
This week’s U.S. data for 12- to 17-year-olds show:
- 21,921 total adverse events, including 1,325 rated as serious and 25 reported deaths. Two of the 25 deaths were suicides.
The most recent death involves a 12-year-old girl (VAERS I.D. 1784945) who died from a respiratory tract hemorrhage 22 days after receiving her first dose of Pfizer’s vaccine.
Another recent death includes a 15-year-old male who died six days after receiving his first dose of Pfizer’s COVID vaccine. According to his VAERS report (VAERS I.D. 1764974), the previously healthy teen complained of brief unilateral shoulder pain five days after receiving his COVID vaccine.
The next day he played with two friends at a community pond, swung on a rope swing, flipped into the air, and landed in the water feet first. He surfaced, laughed and told his friends “Wow, that hurt!” He then swam toward shore underwater, as was his usual routine, but did not re-emerge.
An autopsy showed no external indication of a head injury, but there was a small subgaleal hemorrhage — a rare, but lethal bleeding disorder — over the left occiput. In addition, the boy had a mildly elevated cardiac mass, increased left ventricular wall thickness and small foci of myocardial inflammation of the lateral wall of the left ventricle with myocyte necrosis consistent with myocardial infarction.
- 57 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 535 reports of myocarditis and pericarditis (heart inflammation) with 527 cases attributed to Pfizer’s vaccine.
- 119 reports of blood-clotting disorders, with all cases attributed to Pfizer.
This week’s U.S. VAERS data, from Dec. 14, 2020, to Oct. 15, 2021, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 54% of those who died were male, 42% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 72.7.
- Of the 3,014 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 41% to Moderna and 8% to J&J.
- 666 reports of Guillain-Barré syndrome, with 40% of cases attributed to Pfizer, 31% to Moderna and 28% to J&J.
- 2,010 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 10,290 reports of blood clotting disorders. Of those, 4,488 reports were attributed to Pfizer, 3,709 reports to Moderna and 2,040 reports to J&J.
- 2,878 cases of myocarditis and pericarditis with 1,815 cases attributed to Pfizer, 939 cases to Moderna and 114 cases to J&J’s COVID vaccine.
Michigan woman died from blood clots after J&J vaccine, autopsy confirms
A 60-year-old woman died from blood clots after receiving J&J’s COVID vaccine, according to an autopsy report released Sept. 20 by Dr. Michael Caplan, a forensic pathologist at Michigan Medicine.
Sandra Jacobs “appears to have succumbed” to a “rare but nevertheless documented” complication associated with the viral vector vaccine — cerebral venous sinus thrombosis (CVST) — Caplan wrote in the summary. This condition brought about “hemorrhagic cerebral infarct,” or stroke caused by brain bleeding, and brain swelling, Caplan wrote.
The death certificate listed the cause of death as “complications of cerebral venous sinus thrombosis” and “recent administration” of a COVID vaccine as the contributing condition.
Caplan deemed the manner of death “natural,” but said it may also be considered a “therapeutic complication” since this is a known vaccine issue. Under “final diagnosis,” Caplan first listed the COVID vaccine.
Jacobs died 13 days after receiving the single-dose J&J vaccine at a CVS pharmacy on April 8 — just five days before federal health agencies temporarily paused the vaccine while they examined an unusual blood-clotting disorder.
FDA clears Moderna and J&J COVID vaccine boosters, allows ‘mix-and-match’ shots
The U.S. Food and Drug Administration (FDA) on Wednesday authorized booster doses of both J&J’s and Moderna’s COVID vaccines, CNBC reported. U.S. regulators also authorized “mixing and matching” vaccines — allowing Americans to get a booster of a different COVID vaccine than their initial doses.
In an email to The Defender, Dr. Brian Hooker Ph.D., P.E., Children’s Health Defense chief scientific officer and professor of biology at Simpson University said:
“To my best knowledge, the data that FDA used to make these assertions [that mix-and-match boosters are safe] doesn’t exist. Israel, which has the most aggressive booster program in the world, distributes Pfizer vaccines only. At this point, my strong assumption is that they’re doing anything and everything they can to assure vaccine compliance, despite the huge numbers of vaccine injuries reported on VAERS.”
The FDA’s decision was handed off to the CDC’s vaccine advisory committee, which met Thursday to discuss Moderna’s and J&J’s booster data. Dr. Rochelle Walensky, CDC director, endorsed the advisory committee’s recommendations, expanding eligibility for COVID booster shots to include people:
- 65 years and older.
- Age 18+ who live in long-term care settings.
- Age 18+ who have underlying medical conditions.
- Age 18+ who work or live in high-risk settings.
For the nearly 15 million people who got the J&J shot, booster doses are recommended for those who are 18 and older and who were vaccinated two or more months ago.
According to CDC data, the most common side effects reported after getting a third shot of Pfizer or Moderna were pain at the injection site, fatigue, muscle pain, headache and fever, followed by chills and nausea.
The data available for J&J was more limited, but people reported fever, fatigue and headache after receiving a second dose, according to the agency.
White House, CDC prepare to vaccinate 5- to 11-year-olds prior to FDA authorization
The White House, on Oct. 20, unveiled plans to roll out COVID vaccines for children ages 5 to 11, even though vaccine safety experts — who advise U.S. drug regulators and review safety and efficacy data — have not yet met to discuss whether Pfizer’s COVID vaccine should be authorized for use in the pediatric age group.
The Biden administration said it will secure enough vaccine doses to vaccinate the 28 million children ages 5 to 11 who would become eligible if the vaccine is authorized for that age group.
The White House will also help equip more than 25,000 pediatric and primary care offices, hundreds of community health centers and rural health clinics and thousands of pharmacies to administer the shot.
The CDC last week issued guidance outlining key aspects of a COVID vaccination program for children younger than 12 years old “designed to inform jurisdictional planning under the assumption of FDA authorization and CDC recommendations of at least one COVID-19 vaccine product for children of this age.”
Beginning Oct. 20, states and other jurisdictions could preorder doses of the Pfizer-BioNTech COVID vaccine formulated for children ages 5 to 11, in anticipation of a rollout that could begin as early as Nov. 3.
FDA delays decision on Moderna vaccine for adolescents citing heart problems
The FDA is delaying a decision on authorizing Moderna’s COVID vaccine for adolescents to assess whether the shot may lead to a heightened risk of a rare inflammatory heart condition, according to the Wall Street Journal.
The FDA’s review of Moderna’s application is ongoing, an FDA spokesperson told Reuters, adding that while the agency cannot predict how long the process may take, it is evaluating the data as expeditiously as possible.
In June, the FDA added a warning to the literature accompanying Pfizer and Moderna mRNA COVID vaccines, to indicate an increased risk of myocarditis. However in May, a few weeks before the FDA added the warning, the agency authorized Pfizer’s COVID vaccine for ages 12 to 17, despite the known risk of myocarditis.
Scientists concerned mRNA boosters could cause heart inflammation in young adults
The risk of mRNA COVID booster shots causing heart inflammation in young adults continues to worry top scientists, Dr. Ofer Levy, director of the Precision Vaccines Program at Boston Children’s Hospital and voting member of the FDA’s advisory panel, said last week.
Some committee members voiced concern about authorizing a third mRNA dose for people 12 and older, due to the risk of two rare heart inflammation conditions — myocarditis and pericarditis.
Levy said younger age groups are less at personal risk of severe COVID, but “somewhat more at risk of this inflammatory heart condition with mRNA vaccines.”
“So it’s a risk-benefit analysis, and that’s why you’re seeing that deliberation,” Levy told CNBC’s Closing Bell.
French health regulator suspends Moderna boosters over myocarditis concerns
As The Defender reported, France’s health regulator said Oct. 15, it would no longer allow booster doses of Moderna’s COVID vaccine. Only Pfizer-BioNTech’s vaccine will be used for the booster campaign moving forward.
The new recommendation applies to people over 65, immunocompromised people and their relatives and people at risk — including healthcare workers. The announcement came amid reports that Sweden, Denmark, Norway, Iceland and Finland suspended use of Moderna’s COVID vaccine for certain age groups over reports of heart inflammation.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.