Thank you for reading and sharing Bailiwick News by email and social media. To support Bailiwick with a paid subscription: Labeling deceits and omissions and fake informed consent for vaccines and other legalized biological and chemical weapons.Analysis of South Carolina's S.54: Medical Informed Consent ActOrientation for new readers; American Domestic Bioterrorism Program; Tools for dismantling kill box anti-law Notes: Labeling deceit and labeling omissions are about labeling biological products, including vaccines, with ill-defined words and arbitrary units of measure and units of potency to deceive users into holding false beliefs: that package contents are identifiable, measurable, stable, non-toxic and therapeutic. Labeling deceit and omissions represent only a small fraction of the many layers of legalized deceit and omissions that have comprised biological product manufacturing, communicable disease control, and vaccination law at any given time since 1902 and up to the present moment. Pulling on any thread of deceit, in any layer at any time, leads to other layers of deceit. It's not possible to fully describe any layer, in any single report. I encourage readers who want to better understand other layers of deceit surrounding any word, phrase, unit of measure, or unit of potency described below or in other Bailiwick reporting, to pull the threads. Deepen vaccine hostility. Help more people stop taking vaccines and stop vaccinating babies and children. Pray the Rosary. A Medical Informed Consent Act bill (S. 54) was introduced in the South Carolina state Senate on Jan. 14, 2025, proposing amendments to South Carolina's current Emergency Health Powers law (Title 44, Chapter 4, enacted in 2002) and related provisions in other sections of South Carolina state law. I looked at the draft Medical Informed Consent Act, focusing on proposed changes to SC 44-4-520, Vaccinations and Treatment. Based on my knowledge of how federal law addresses biological product and vaccine package labeling and informed consent to enable and hide intentional poisoning programs by disguising poisoning campaigns as regulated pharmaceutical product manufacturing, distribution and use, I think people who are knowledgeable about how to neutralize this state law proposal, neutralized it before introduction. It would still be good if it passed, if only because other amendments proposed by S.54 would require notification to product recipients that they and their survivors can't prosecute or sue anybody if they're injured or killed, by defining the term Indemnified product.
That's notification by South Carolina lawmakers, to South Carolina people, that US-HHS, US-DoD, US-FDA, US-NIH, US-CDC, US-ASPR, US-BARDA, US-DARPA, Pfizer, Moderna, National Resilience, Rentschler, and South Carolina pharmacists, nurses and doctors are all jointly licensed to maim and kill using all vaccines (which are all indemnified products) and countermeasures, under biological product laws that legalize manufacture of biological and chemical weapons, deceptive labeling of packages, and use of the products to torture, maim and murder recipients. When people understand that FDA licenses, authorizations and approvals for manufacture and use of indemnified products are licenses to kill, those people stop taking vaccines and stop vaccinating babies and children. Analysis The current South Carolina law relating to Vaccinations and treatment (S.C. Code 44-4-520, adopted in 2002), does not include a section defining informed consent. S.C. Code 44-4-520 currently reads:
With S.54, South Carolina lawmakers are proposing to add informed consent provisions to S.C. Code 44-4-520.
The proposed S.54 amendments would also add a new provision to Section 44-4-520 on "safety and efficacy" review and "adverse event monitoring."
The proposed South Carolina state law language about informed consent for vaccination and treatment mirrors federal law for all biological products since 1902 and for EUA countermeasures since 2003. Federal biological product and EUA laws are written to exempt manufacturers and regulators from having to label products with information about the specific identity and quantity (mass, volume, concentration) of biological material inside packages of biological products. For biological products (all vaccines) and for EUA products, proper name, manufacturer name and address, and general descriptions are the only enforceable and enforced legal requirements. South Carolina lawmakers will, if S.54 passes, embed this limited general information format, mirroring federal law in state law, by limiting the required information to "an explanation of the vaccine or treatment that is written in language that is understandable to the average lay person." This is related to what Sasha Latypova and I have pointed out: it's not possible for anyone to give informed consent without having specific information about what, exactly, is in the individual package presented to a specific recipient at a specific time and place. Biological product labeling laws are written to require general, but not specific information, because the specific contents of any given package of biological material at any given moment in time, cannot be specifically known, identified or fully characterized at all. Scientific identification and measurement methods are capable of only partial characterization of biological organisms and living systems not because of limits to scientific knowledge or technology that may someday be overcome, but because of the inherent variety and instability of biological organisms themselves. Assessment methods destroy the samples to obtain the limited information that can be gleaned. Each aliquot is differs from each other aliquot, even those drawn from the same batch. And each aliquot differs from itself at earlier or later moments in its existence. It's also related to what Sasha Latypova and Mike Yeadon have both pointed out: there is no way to establish any "dose" by mass or volume of injected biological material, because all injected biological material interacts in a unique way and for an unpredictable, indeterminate amount of time with the biological processes of each specific recipient. Living organisms are in a state of constant change. They're born and they grow. They take in nutrients, use energy, change form, excrete waste. They communicate and cooperate with other living organisms. They decay, fragment and die. At any given moment all along the biological product propagation and manufacturing chain, all of those processes are underway in vaccine batches and bottles. The biological events unfold at active, rapid speeds when the living organisms are at room temperature or body temperature within a laboratory processing container. The events occur at slower rates when the living organisms are suspended, encapsulated, refrigerated or frozen. The events resume — rapidly again — when the living organisms are defrosted, diluted, warmed, mixed and injected into another, larger living organism. Since there's no way for any public health-military officer, manufacturer, regulator or vaccinator to know the specific identity of what's in any package at any given time, because biological products are unstable mixtures of living and non-living matter, and no way to predict how each specific living body will respond to the material after injection, there's no way for any of them to tell the recipient what the product is or what effect it will have. Poisoners among US public health, military, scientific, medical, legal and financial officers have known these facts from the beginning of the modern vaccination era. That's why they have written and executed laws to exclude identity information from product labels for all viruses, serums, toxins, antitoxins and vaccines since the virus-toxin law enacted by Congress in 1902 [see below, section titled Leapfrogging Mutual Exemptions] and also from all labels and "fact sheets" for EUA products since the emergency countermeasures law enacted by Congress in 2003. Oct. 22, 2020 FDA VRBPAC meeting: Marion Gruber, Director, FDA Center for Biologics Evaluation and Research CBER Office of Vaccines Research and Review (OVRR), transcript at p. 37:
Gruber was correctly reporting on the legal requirements: regulators and manufacturers are legally-authorized to omit specific identity information from EUA product fact sheets, which are substitute or false informed consent documents containing only general information, descriptions, or explanations. Congress enacted the omission provision in 2003 (PL 108-136, NDAA FY2004 at 117 Stat. 1686) and the provisions entered into the Food Drug and Cosmetic Act (US Code Title 21 at 21 USC 360bbb-3(e) Congress enacted substitute or fake informed consent provisions to continue to hide labeling omissions; to continue to hide the non-existence of legal requirements, since 1902, that biological product manufacturers and regulators identify and publish (on labels and other printed material) the specific contents of vaccine and other biological product packages.
Emergency Use Authorization letters for COVID-19 vaccines corroborate the legalized omission of specific identity information from product labels. See, for example, Dec. 11, 2020 letter, Denise Hinton, FDA to Elisa Harkins, Pfizer, published at 86 FR 5204:
The December 2030 Product Description by FDA and Pfizer-BioNTech uses the indefinite article "a nucleoside-modified messenger RNA..." and provides no information about how to validate the claimed mass and concentration of an indeterminate product (30 mcg per 0.3 mL dose) or convert from the claimed mass and concentration of the indeterminate biological product to predictable effects. Again, this is because mass and concentration of unstable, dynamic mixtures of living and dying organisms and fragments of their cells cannot be validated — there are no scientific techniques, equipment or methods that don't fully destroy each sample — and because effects cannot be predicted accurately and specifically: effects vary immeasurably through the interaction of the injected material with itself before injection and with the recipient's own living body after injection. FDA, Pfizer and vaccinators provide no information about the specific molecular structure of the claimed RNA molecules, because RNA molecules are not stable and, if present at all, are present in a variety of ever-changing forms in the package, interacting with, transforming and being transformed by all the other listed contents, which also may or may not be in the bottle at any given moment while the contents are active, growing, living and decaying. The Dec. 2020 Fact Sheet given to recipients includes a list of names of substances, including unspecified "mRNA."
Publicly-available manufacturing contracts, such as the Pfizer Statement of Work produced during Brook Jackson’s False Claims Act case, and publicly-available regulatory application documents redact all information that would further reveal the unstable, mixed, unspecified, unidentifiable nature of vaccine contents.
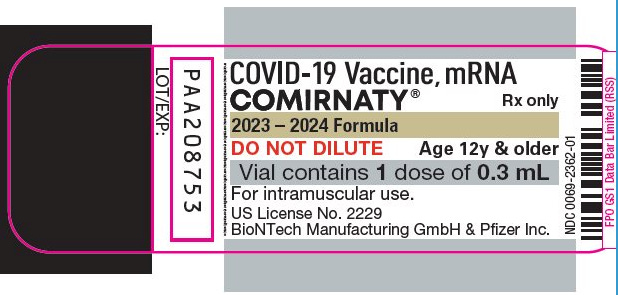
Even after the fake FDA "approval" of the Pfizer-BioNTech Biologics License Application (BLA) in August 2021, for example, the Comirnaty box for the vial simply describes the contents as "one dose of 0.3 mL." Screenshot from FDA Purple Book, Comirnaty entry, 2023-2024 Formula Some records:
Leapfrogging mutual exemptions Tracking the two separate legal pathways for "biological products" and for "drugs" from the opening of the legal hole for biological products through 1944. BIOLOGICAL PRODUCTS - Viruses, serums, toxins, antitoxins and analogous products, including all vaccines. 1902 Virus, Serum and Toxin Act, PL 57-244 Congress required package labels for viruses, serums, toxins, antitoxins and analogous products - to contain:
Congress required no information about product identity, mass, volume or other physical or chemical qualities or quantities. That was the opening of the legal hole through which unidentified, unidentifiable, mixtures of unstable, foreign biological substances would be legally labeled as medicines and legally injected into people to poison, sicken and kill them for the following 120+ years. DRUGS 1906 Pure Food and Drug Act, PL 59-384 Section 6 defined the term "drug" as "all medicines and preparations recognized in the United States Pharmacopeia-National Formulary [USP-NF] for internal or external use, and any substance or mixture of substances intended to be used for the cure, mitigation, or prevention of disease of either man or other animals." Section 7 provided that, for drugs sold under USP-NF-recognized names, a drug would be deemed adulterated under either of two conditions:
Section 8 defined misbranded as applying to all drugs "the package or label of which shall bear any statement...regarding such article, or the ingredients or substances contained therein which shall be false or misleading in any particular." The 1906 Pure Food and Drug Act also deemed drugs misbranded if demonstrated to be "an imitation of or offered for sale under the name of another article;" in packages that had had original contents removed and substituted with other contents; or if the package label failed to list the "quantity or proportion of any alcohol, morphine, opium, cocaine, heroin, alpha or beta eucaine, chloroform, cannabis indica, chloral hydrate, or acetanilide, or any derivative or preparation of any such substances." Unpacking the resulting status of biological products under the Pure Food and Drug Act: Biological products were not sold under names recognized by the USP-NF, and — as biological products subject only to the 1902 law — were not required to be labeled with any specific, identifying information about ingredients or substances. Since there were no specific "statements about the article, ingredients or substances" on biological products at all, there were no statements that could be assessed or deemed "false or misleading." Adulteration and misbranding provisions were therefore not applicable to biological products. DRUGS 1938 Federal Food, Drug and Cosmetic Act - (PL 75-717) Congress in 1938 passed the Federal Food Drug and Cosmetic Act (FDCA), repealing and replacing the 1906 Pure Food and Drug Act, and carrying forward the comprehensive inapplicability of drug manufacturing, labeling and distribution regulations to biological product manufacturing, labeling and distribution activity, through Sec. 902(c):
For drugs already in use, the FDCA maintained most of the 1906 rules for drugs. The 1938 FDCA deemed a drug sold under a USP-recognized name to be adulterated if
For drugs already in use, 1938 FDCA required labels to contain:
For drugs already in use, 1938 FDCA deemed a drug to be misbranded if its labeling was:
The 1938 FDCA also provided for several exemptions and waivers to be promulgated by the Secretary of Agriculture at his discretion, including conditions under which any otherwise applicable requirement "is not necessary for the protection of the public health." For new drugs — drugs not already in use by 1938, that manufacturers wanted to begin selling — the 1938 FDCA required applicants to provide the Secretary of Agriculture with
New drug applications would become effective, allowing introduction of the drug into interstate commerce, automatically on the sixtieth day after filing, unless the Secretary issued an order refusing to permit the application to become effective. For new drug applications, the 1938 FDCA authorized the Secretary to issue an order refusing to permit the application to become effective, upon finding
BIOLOGICAL PRODUCTS 1944 Public Health Service Act (PL 78-410) With the 1944 Public Health Service Act, Congress reinforced the separation of biological product regulation from drug regulation that had already been put in place in 1902 and 1906 and reinforced in 1938:
Through the 1944 PHSA, Congress added to the list of biological products, which up until then included "virus, therapeutic serum, toxin, antitoxin or analogous product" the phrase "arsphenamine or its derivatives (or any other trivalent organic arsenic compound)." PHSA 351(a) Through the 1944 PHSA, Congress left in place the very short list of required package markings, limited to:
Through the 1944 PHSA, Congress left in place the legal hole through which poisons could be manufactured, labeled without specific identification or quantification of contents, distributed and used, disguised as standardized, regulated medicinal products. At Section 351(d) Congress introduced the phrase "continued safety, purity, and potency" falsely described as "standards" biological product manufacturing processes must be "designed to insure" for licenses to be issued, even though no such standards had ever existed, and no manufacturing, testing or regulatory compliance procedures had ever been designed to insure them. Such standards can never exist, and such manufacturing processes can never be designed or used, because biologically-active biological organisms cannot be stabilized, standardized, purified, or rendered safe to inject into another living creature, or rendered potent or effective for anything other than untraceable poisoning. Related:
All content is free to all readers. All support — reading, sharing and financial — is deeply appreciated. |

No comments:
Post a Comment