Carol Taccetta, MD: “Boxed Warning,” the FDA’s Most Serious Warning, Is Absent from All COVID-19 Vaccine Fact Sheets
Introduction
There are some adverse reactions in a product label that are so serious, and possibly even preventable, that special labeling is required to highlight this warning information: a “BOXED WARNING.”
![]()
https://www.fda.gov/media/71866/download
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57
This boxed warning information, “critical for a prescriber to consider,” is commonly referred to as a “black box.” (https://www.fda.gov/media/71866/download)
Boxed Warnings are absent from the labeling of all three currently available COVID-19 vaccines in the United States. In the letter below, I write to the Food and Drug Administration (FDA), requesting that certain CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS “rise to the level of a Boxed Warning.” (https://www.fda.gov/media/71866/download and https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products)
As an aside: During my research, I noted that, despite their differing platforms (mRNA vs. protein subunit-based), all three vaccines share the same five warnings, suggesting a common denominator, such as being spike-protein based. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html)
Dr. Taccetta’s Letter to Dr. Peter Marks, Director of CBER at the U.S. FDA
July 23, 2023
Peter Marks, MD, PhD
Director, Center for Biologics Evaluation and Research
U.S. Food and Drug Administration
10903 New Hampshire Avenue
W071-7232
Silver Spring, MD 20993
mailto:Peter.Marks@fda.hhs.gov
Dear Dr. Marks,
Based on my experience of working with the FDA for 25+ years in drug safety, and in light of the FDA’s own guidance of 2011, I interpret the COVID-19 vaccine warning information under CONTRAINDICATIONS, as well as “Management of Acute Allergic Reactions” and “Myocarditis and Pericarditis” under WARNINGS AND PRECAUTIONS as all rising to the level of a Boxed Warning. (https://www.fda.gov/media/71866/download)
Please note: In my assessment, there are many other “observed serious adverse reactions” which are not mentioned in the current product labeling: my arguments below are solely based on the information currently acknowledged by the FDA in the Fact Sheet Labeling (and its hyperlinks) as a stand-alone document.
The Boxed Warning guidance applies to emergency use authorization (EUA) products, as well as approved products, as seen in this recently revised EUA Paxlovid Fact Sheet:
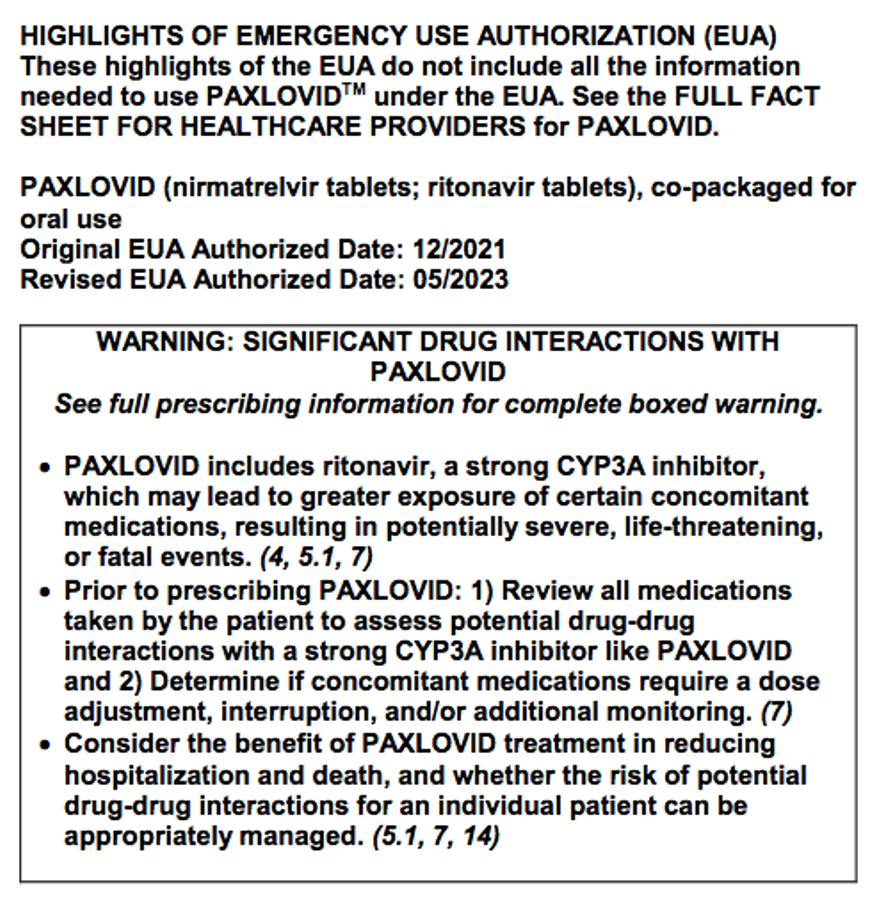
https://labeling.pfizer.com/ShowLabeling.aspx?id=16474&format=pdf
Today, there are three COVID-19 vaccines available in the U.S., each under EUA only:
Under Section 4, CONTRAINDICATIONS, each manufacturer contraindicates use of their respective vaccine in individuals with a known history of severe allergic reaction to a component in their own vaccine. Also, each fact sheet carries the same five warnings under Section 5, WARNINGS AND PRECAUTIONS: 5.1 Management of Acute Allergic Reactions, 5.2 Myocarditis and Pericarditis, 5.3 Syncope, 5.4 Altered Immunocompetence and 5.5 Limitations of Vaccine Effectiveness. (https://www.fda.gov/media/167211/download, https://www.fda.gov/media/167208/download, and https://www.fda.gov/media/159897/download)
As you know, a “Boxed Warning” should appear in the product’s label if warning information under WARNINGS AND PRECAUTIONS or CONTRAINDICATIONS meets one of the following criteria:

https://www.fda.gov/media/71866/download
In my assessment, the following contraindication under CONTRAINDICATIONS and two warnings under WARNINGS AND PRECAUTIONS rise to the level of a Boxed Warning:
Section 4 CONTRAINDICATIONS
Under Section 4, CONTRAINDICATIONS, each respective fact sheet states:
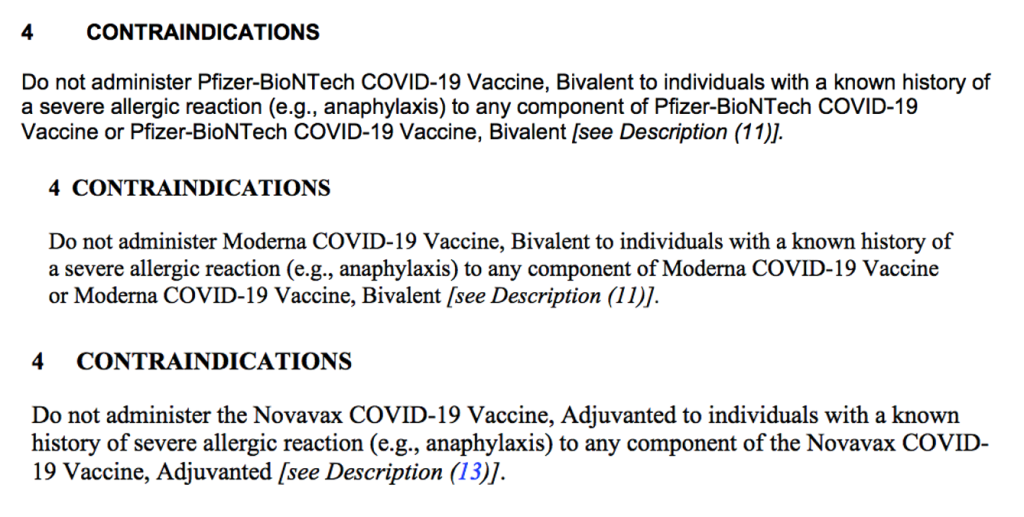
Boxed Warning criterion met include:
- “Infrequently, a boxed warning can also be used in other situations to highlight warning information that is especially important to the prescriber (e.g., reduced effectiveness in certain patient populations). Information included in the WARNINGS AND PRECAUTIONS and CONTRAINDICATIONS sections should therefore be evaluated to determine whether it warrants inclusion in a boxed warning.”
ARGUMENT:
Although the current warning information under CONTRAINDICATIONS for all three vaccines is accurate, a Boxed Warning would highlight, front and center, this critical information to vaccine providers. The current fact sheets first mention the contraindication only on page two or three of the “FACT SHEET FOR HEALTHCARE PROVIDERS [VACCINATION PROVIDERS].
Section 5.1 Management of Acute Allergic Reactions
Under Section 5.1, WARNINGS AND PRECAUTIONS, each respective fact sheet states:

Boxed Warning criteria met include:
- “There is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug”
ARGUMENT:
Following the link within WARNINGS AND PRECAUTIONS, the CDC acknowledges anaphylaxis as “life threatening”:

https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html
-and-
- “There is a serious adverse reaction that can be prevented or reduced in frequency or severity by appropriate use of the drug (e.g., patient selection, careful monitoring, avoiding certain concomitant therapy, addition of another drug or managing patients in a specific manner, avoiding use in a specific clinical situation)”
ARGUMENT:
Anaphylaxis is a “serious adverse reaction that can be prevented or reduced in frequency or severity by appropriate use of the drug”:
- See CONTRAINDICATIONS regarding “avoiding use in a specific clinical situation” and CDC link within WARNINGS AND PRECAUTIONS regarding initial assessment, i.e., “patient selection”
- As in WARNINGS AND PRECAUTIONS, recipients must be “monitor[ed]…for the occurrence of immediate adverse reactions,” i.e., “careful monitoring”
- As per CDC link within WARNINGS AND PRECAUTIONS, “Addition of another drug” (i.e., patient’s home epinephrine autoinjector) may be necessary to prevent a serious outcome
- As in WARNINGS AND PRECAUTIONS, “Appropriate medical treatment … must be immediately available” to “manag[e] patients in a specific manner”
(https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html)
Section 5.2 Myocarditis and Pericarditis
Under Section 5.2, WARNINGS AND PRECAUTIONS, each respective fact sheet states:

Boxed Warning criteria met include:
- “There is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug”
ARGUMENT:
Although a seriousness outcome for myocarditis/pericarditis is not mentioned in the labeling, it is noted that “some cases required intensive care support,” suggesting a potentially fatal, life-threatening or permanently disabling outcome.
-and-
- “There is a serious adverse reaction that can be prevented or reduced in frequency or severity by appropriate use of the drug (e.g., patient selection, careful monitoring, avoiding certain concomitant therapy, addition of another drug or managing patients in a specific manner, avoiding use in a specific clinical situation)”
ARGUMENT:
Myocarditis and pericarditis are “serious adverse reaction[s] that can be prevented or reduced in frequency or severity by appropriate use of the drug”:
- As in WARNINGS AND PRECAUTIONS and CDC link within WARNINGS AND PRECAUTIONS, “patient selection” is critical, such as in screening for a “history of myocarditis or pericarditis,” as well as age or gender-related risk.
- As in WARNINGS AND PRECAUTIONS and CDC link within WARNINGS AND PRECAUTIONS, “careful monitoring,” “particularly within the first week following vaccination,” involves assessing for specific myocarditis/pericarditis symptoms in “adolescents and young adults” and non-specific symptoms in “younger children.”
- As per CDC link within WARNINGS AND PRECAUTIONS, “managing patients in a specific manner” for post-covid-19 vaccine myocarditis/pericarditis is outlined, as is adjusting vaccine dosing interval to 8 weeks in “some people,” and adjusting dosing interval in those receiving both covid-19 and orthopoxvirus vaccines.
- As per CDC link within WARNINGS AND PRECAUTIONS, “avoiding use in a specific clinical situation” applies to those with a “history of myocarditis or pericarditis after a dose of any COVID-19 vaccine” in which case, “[a] subsequent dose of any COVID-19 vaccine should generally be avoided.”
(https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html and https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html – myocarditis-pericarditis)
I look forward to your urgent action on adding the warning information from CONTRAINDICATIONS and “Management of Acute Allergic Reactions” and “Myocarditis and Pericarditis” under WARNINGS AND PRECAUTIONS to a Boxed Warning.
Sincerely,
Carol Taccetta, MD, FCAP
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.

No comments:
Post a Comment