Report 73: Pfizer Knew by November 2020 That Its mRNA COVID Vaccine Was Neither Safe Nor Effective. Here Is What Pfizer’s Employees and Contractors Knew and When They Knew It.
Introduction
Through the review of two documents – Pharmacovigilance Plan for Biologic License Application #125742 Of Covid-19 mRNA vaccine (nucleoside modified) (BNT162b2, PF-07302048) and 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021 – referred to below as “PV” and “5.3.6,” the contributors to this report came to understand Pfizer knows its product does not work and that it poses a danger to the public. In this report, they have demonstrated these admissions using Pfizer’s own words. When those documents are overlaid with the Emergency Use Authorization (EUA) from 2020 and the EUA from late 2021, it becomes apparent that the Company ignored safety signals and used weak statistics to justify product use. When these documents are viewed together, there is sufficient evidence to say Pfizer understood that there were problems with its mRNA COVID product before the original EUA was submitted in November 2020.
Abbreviations
PV = Pharmacovigilance Plan for Biologic License Application #125742 Of Covid-19 mRNA vaccine (nucleoside modified) (BNT162b2, PF-07302048). Date of Report: 28 July 2021, Version 1.1
EUA 2020 = Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum. Date of Document: 20 November 2020, Author: Marion F. Gruber, Ph.D., Director, CBER/OVRR
5.3.6 = Reissue of 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021. Approval Date: 30 April 2021.
EUA 5-11 = Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum. Date of Document: 06 October 2021, Author: Peter Marks, M.D., Ph. D., Director, CBER, and Acting Director, CBER/OVRR.
SOC = System Organ Class
AE = Adverse Event
Executive Summary in chronological order:
- In November 2020 (EUA 2020), Pfizer dismissed safety signals in its clinical trial C4591001 (ages 16+). Moreover, although Pfizer considered any adverse event (AE) within six weeks of product use to be reasonably associated with the product (EUA 2020, p. 10), it dismissed the observed safety signals in EUA 2020, 5.3.6, PV, and EUA 5-11.
- In November 2020 (EUA 2020), Pfizer had a weak demonstration of efficacy based on very few occurrences (eight cases versus 162 cases). C4591001 was essentially invalid because investigators failed to confirm or deny 3,410 suspected COVID cases (1,594 vaccinated and 1,816 placebo). If COVID occurred in the thousands and investigators used only 170 cases for efficacy, their statistics did not reflect reality. Investigators then destroyed their clinical trial by unblinding and vaccinating all placebo cohort participants (PV, p. 13, pp. 18-19). In effect, this act terminated the trial. Pfizer acknowledged unblinding and vaccinating the placebo cohort would adversely affect the data (EUA 2020, p. 53). The company cut off data collection the day after placebo participants were vaccinated (EUA 5-11, p. 12).
- Through December 2020 to February 2021 (5.3.6) field reports, Pfizer observed AEs including deaths and permanent harms. Per Pfizer’s own standard of AEs within six weeks of product use being considered product-related (EUA 2020, p. 10), Pfizer de facto recognized its product caused AEs, because many of the AEs in 5.3.6 occurred within hours or days of product use.
- In its report dated July 28, 2021 (PV), Pfizer still planned to use C4591001 (a portion of which was due April 2023) to reach final conclusions on its mRNA COVID product’s efficacy and safety. The cut off of data collection on March 12, 2021, should be understood as Pfizer’s acknowledgement of the termination of its clinical trial. Pfizer attempted to substitute titer-based lab tests for efficacy, but later admitted lab titers do not represent disease protection (i.e., efficacy) (EUA 5-11, p. 13).
- In Pfizer’s July 2021 report (PV), Pfizer acknowledged pericarditis and myocarditis as risks of product use. Pfizer did not call it a dose-response, but it reported pericarditis and myocarditis risks as higher after dose #2 (PV, p. 50). Pfizer reported a similar dose-dependent pattern elsewhere (EUA 2020, p. 6, p. 42, p. 56; EUA 5-11, p. 46). All other AEs noted in the EUA 2020, from study C4591001, and AEs reported from the field in 5.3.6 were ignored. Additional studies listed by Pfizer in PV seem to not exist online.
- In October 2021 (EUA 5-11), efficacy was weakly demonstrated. Investigators did not draw upon C4591001 for support. Rather, they substituted titers for efficacy.
- In Pfizer’s October 2021 EUA 5-11 submission, Pfizer described a dose-response relationship between its product and AEs in both dosage and dose number. Investigators speculated that subclinical damages would manifest in the long-term. The implication is that continued doses with subclinical damages would eventually manifest as clinical damages. Pfizer admitted a young male subject’s AE, previously dismissed, was actually related to product use months after initial signal detection. This event represented a pattern of behavior: no matter what AE occurred, investigators concluded it was unrelated to Pfizer’s product.
- EUA 5-11 introduced unsupported points to push product use in children. Pfizer introduced claims on transmission prevention and attacked the unvaccinated. Investigators did not provide clinical trial evidence for support. The product did not have well-demonstrated benefits, so any risks (and there are many) immediately rendered a poor risk-benefit ratio.
Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum. Date of Document: 20 November 2020, Author: Marion F. Gruber, Ph.D., Director, CBER/OVRR.
EUA 2020 Regarding Efficacy
Pfizer’s original efficacy claim was based upon ratios between very small numbers over a short period of time (six weeks), representing extremely weak evidence. The vaccinated group had eight COVID-19 cases, and the placebo group had 162 cases (EUA 2020, p. 20). Investigators used this simple ratio to determine high efficacy, as 162 is around 20 times greater than eight. Compare these occurrences against the 17,411 in the vaccine cohort and the 17,511 in the placebo cohort used for the statistical evaluation (EUA 2020, p. 23). Eight and 162 were infinitesimal. If an individual took the vaccine, it dropped their risk of a positive PCR test from 0.92% to 0.045% in a six-week period. To put it another way, one should consider the result as doses needed to treat the population. Investigators vaccinated about 17,500 individuals (35,000 doses) to prevent approximately 150 COVID cases. For the other 17,350, the benefit was effectively zero during the six weeks. For them, vaccination was only risk.
This analysis described the purest meaning of the investigators’ results. They arrived at a statistic derived under a narrow set of parameters, the most important of which was the very short-term nature of six weeks. In this context, the fraction of a percentage drop in COVID risk was inconsequential to the population. Pfizer failed to discuss the alternative conclusions based on few occurrences in a short time span. Pfizer would have understood that 35,000 doses to save about 150 cases was not practical for a public health intervention. This approximation of doses-needed-to-treat is just as valid as the efficacy claim in the context of a six-week period. It is the same result at which Pfizer arrived, drawn from the same evidence; however, it is rephrased in more practical language. A reasonable person would not take an experimental drug if the benefit was a 0.88% drop in COVID risk.
To create strength in statistical evaluation, the trial needed to run for two years to allow occurrences to build up in the placebo and experimental cohorts. Only then could valid conclusions be made. The result would either hold up and become stronger with time as vaccinated participants resisted disease over the long term, or investigators would find that COVID cases also accumulated in the vaccinated cohort just as they did in the placebo cohort. The practical reality was that this short-term cultivation of data was enough to perform a statistical math exercise only. Investigators did not demonstrate 95% efficacy over a year or longer period of time. If efficacy waned in the short-, middle-, or long-terms, it would not be captured by this preliminary analysis. For a short, preliminary, investigative trial with further follow-up planned, Pfizer’s conclusion was technically acceptable, despite issues, as long as the clinical trial continued, unaltered, to the planned 24-month completion date.
On page 41 (EUA 2020), the investigators reported there was a significant issue in their clinical trial, which would have affected even their preliminary efficacy assessment. There were suspected COVID cases numbering in the thousands that were never tested. The authors discussed this finding in the context of safety, discussing both reactogenicity and adverse events, but they failed to provide commentary on efficacy.
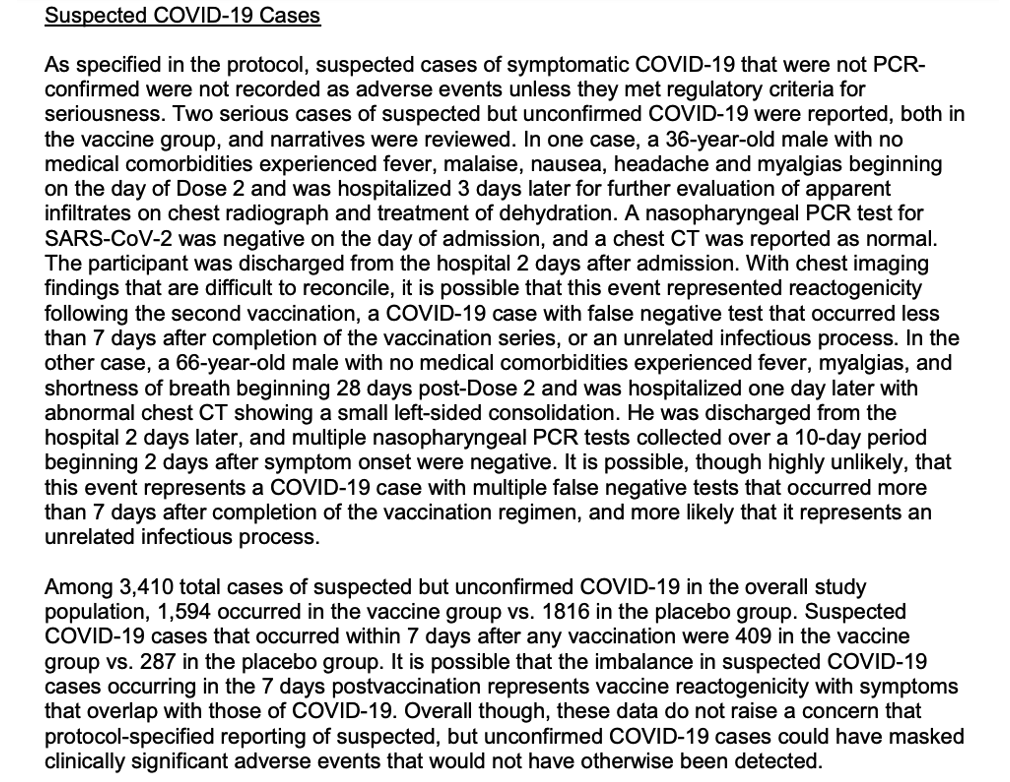
https://www.fda.gov/media/144416/download, p. 41.
They unwittingly admitted in this section that they did NOT test large numbers of participants with suspected cases of COVID. Since testing was a critical procedure to determine efficacy, it brings serious questions to the legitimacy of the clinical trial. Based on this information, the EUA clinical trial C4591001 results were not valid. This conclusion should stand until personnel operating these trials provide important context and relevant information proving otherwise.
The EUA 2020: Implications of Failure to Test Suspected COVID-19 Cases
Investigators reported 1,595 suspected COVID cases in the vaccine group and another 1,816 suspected COVID cases in the placebo group (EUA 2020, p. 41). Remember, investigators determined efficacy on 170 total COVID cases between the cohorts. If they thought they had thousands of other COVID cases and never confirmed them through testing, they not only violated Pfizer’s protocol but also failed to include cases necessary to reach the correct determination of efficacy. If what the investigators reported was true, the C4591001 study would have been invalid by November 2020. The section to follow will highlight the implications of this testing problem regarding efficacy.
If the investigators were correct about missing COVID cases and these 3,410 cases failed to make their analysis due to their failure to test, the real comparison could have been 1,602 vaccinated against 1,978 placebos. The risk to placebo participants could have been 11.3% compared to 9.2% in the vaccinated cohort for a 2.1% drop in risk of COVID. Practically speaking, it would not be a great difference in scale between the occurrences between the cohorts. Most importantly, their efficacy would be closer to 19% with these numbers. Consider how this incidence rate would affect the clinical trial. If investigators witnessed thousands of cases of COVID in both cohorts in this short period, they were on track to run out of trial participants in about a year if it continued. Efficacy in that scenario would approach zero, and investigators would have been able to see that inevitability coming if thousands were getting COVID in both cohorts.
The true efficacy could be 95%, 19%, 0%, or some other figure. Hypothetically, there could have been more COVID cases in the vaccinated group, which would have represented negative efficacy. We cannot know because the investigators did not follow up on suspected cases with testing. It begs the question of how they decided who and when to test. The failure to test thousands of potential cases combined with the arrival at eight vs 162 gives the appearance that investigators cultivated the results. If there is a valid explanation, the public deserves to hear it from the investigators.
EUA 2020 Regarding Safety
The standard for considering AEs to be potentially related to the product are as follows: “From a safety perspective, a 2-month median follow-up following completion of the full vaccination regimen will allow identification of potential adverse events that were not apparent in the immediate post-vaccination period. Adverse events considered plausibly linked to the vaccination generally start within 6 weeks of vaccine receipt” (EUA 2020, p. 10). For reference, the EUA findings from C4591001 represented six weeks of follow-up on average per patient.
In the vaccine group, investigators reported occurrences of myocardial infarction (MI) as 0.02% (four to five patients, p. 40), cerebrovascular accident (CV) as 0.02% (four to five patients, p. 40), appendicitis as 12 patients (0.04%) (p. 40), and Bell’s palsy as four patients (~0.02%) (p. 37). The standard of using few occurrences to make conclusions, as used for efficacy, applied here, too. During the short, six-week study, the risk of MI or CV quadrupled or quintupled in the vaccine group as compared to the one placebo death from MI and the one placebo death from hemorrhagic stroke (EUA 2020, p. 40). Risk of appendicitis increased 50% with vaccination (12 versus eight). Bell’s palsy did not occur in any placebo participants. These observations were safety signals.
Investigators reported six deaths during the trial (two vaccine versus four placebo). One vaccine subject was over 55 and experienced cardiac arrest 62 days after dose #2. The other subject was over 55 and died of unlisted causes three days after dose #1, but investigators noted he was obese with atherosclerotic disease. The placebo deaths were one MI, one hemorrhagic stroke, and two unknown causes. Of these six, one was under 55-years-old, and the specific age is not disclosed. Investigators assured the public that “all deaths represent events that occur in the general population of the age groups where they occurred, at a similar rate” (EUA 2020, p. 40).
The investigators took time in the EUA to declare the AEs as chance events consistent with the general population at large. This acknowledgement is extended to deaths (p. 43), appendicitis (p. 43), and Bell’s palsy (p. 52), yet no commentary accompanies MI and CV. These assertions are not valid per their own standard from page 10 — i.e., “From a safety perspective, a 2-month median follow-up following completion of the full vaccination regimen will allow identification of potential adverse events that were not apparent in the immediate post-vaccination period. Adverse events considered plausibly linked to the vaccination generally start within 6 weeks of vaccine receipt” — where they noted any occurrences within their six-week trial period would be plausibly linked to product use. It was also not valid because the investigators were charged with running a clinical trial where findings from the vaccine group were compared specifically to the placebo group. It was the entire purpose of the clinical trial. Rather than doing this analysis in an open and honest way, the investigators, who realized there could be significant safety issues, blamed chance. Nonetheless, investigators used very small numbers to determine that efficacy was high. They then ignored the same small numbers to determine safety, which included dismissal of adverse events that occurred within a short time after doses. The methods that were good enough for efficacy were suddenly not good enough for safety.
The Fate of the Placebo Cohort
In light of the problems highlighted above with statistics based on small numbers, the investigators had one course of action to pursue truth in their clinical trial. They needed to run the 24-month clinical trial to completion. The missed COVID cases were an issue, but they could potentially make up for it with due diligence by tracking down these cases and by following both cohorts to the two-year completion date. In the event the product worked very well with an excellent safety profile, the evidence over a longer span would tell that truth despite imperfections in the process. It was in the best interests of Pfizer and the world’s patients to witness this truth. If it turned out the product did not work or that it was not safe or both, the integrity of the clinical trial C4591001 was critically important to stop product use.
On page 53 of the EUA 2020, the investigators discussed the consideration to unblind and to vaccinate the placebo cohort. The Vaccines and Related Biological Products Advisory Committee (VRBPAC) provided discussion.
“The committee discussed potential implications of loss of blinded, placebo-controlled follow-up in ongoing trials including how this may impact availability of safety data to support a Biologics License Application (BLA). Some pointed out the importance of long-term safety data for the Pfizer-BioNTech COVID-19 vaccine as it is made using technology not used in previously licensed vaccines. In response to the question whether the ongoing Phase 3 study would still be sufficiently powered if eligible placebo recipients were vaccinated, Pfizer asserted that, even with an anticipated loss of placebo-controlled follow-up of 20%, the study would maintain adequate statistical power and would be positioned to accrue additional data on vaccine efficacy, including efficacy against severe disease, as well as safety, although unblinding of the study would reduce interpretability of results” (Bold Added, EUA 2020, p. 53).
Pfizer already had statistical issues documented above and acknowledged within the EUA 2020 that they were open to reducing their study’s power further by unblinding and vaccinating the placebo cohort participants. There was no rubric for how they would choose which participants would be among the unblinded 20%, but they had a solution in mind. Nonetheless, with this 20% standard established by Pfizer in this November 2020 EUA, Pfizer vaccinated their entire placebo cohort. Pfizer documented it outside the view and knowledge of the world’s patients (Table 5, PV, pp. 18-19). Pfizer reported the vaccination of 19,696 placebo participants, representing the entirety of their placebo cohort. Pfizer completed this process rapidly, finishing on 12 March 2021.
Investigators moved to unblind and vaccinate placebo participants immediately after the EUA 2020 was approved. Per Pfizer’s own 20% standard established in the EUA 2020 (p.53), the power of this study was effectively destroyed on March 12, 2021 (PV, ps.18-19). Thus, Pfizer essentially ended its clinical trial, C4591001, in March 2021. Whatever continued on was something else approximating an observational study. If the product was highly efficacious and safe, it was not in Pfizer’s interest to manipulate the placebo cohort. A complete clinical trial with clean data, free of manipulation, was in the best interest of patients and society, because it was much more likely to conclude the truth. Pfizer committed this act before it had valid efficacy and safety data. As a result, the trial cannot produce an accurate efficacy analysis.
EUA 2020 – Conclusion Summary Statement
By the completion of the EUA 2020, the investigators knew they had significant shortcomings in their efficacy assessment. They had safety signals that they refused to acknowledge as product related. Yet, Pfizer pushed an efficacy statement it could not support and declared a high level of safety that was refuted by its own reported observations. If the limited data were sufficient for efficacy, the same limited data were sufficient to acknowledge significant safety signals. Furthermore, Pfizer’s failure to capture COVID cases in its study cohorts rendered any efficacy outputs invalid. The investigators were subject matter experts in these areas. The construction of statistics in the EUA, combined with selective observations, indicated they very likely knew or at least suspected the product had limited or zero efficacy and significant safety concerns by November 2020. Their termination of the clinical trial before valid data became available did not serve the interest of society; it seemingly served to hide data from the public.
5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021.
FDA Approval Date: 30 April 2021
Obtained by Court Order
5.3.6 Regarding Safety
The 5.3.6 document (38 pages) was a safety-monitoring report authored by Worldwide Safety at Pfizer (WSP). The findings represented adverse events submitted voluntarily to Pfizer’s safety database from various sources, including medical providers and clinical studies, between 01 December 2020 and 28 February 2021. The AEs consisted of 42,086 cases reporting 158,895 total adverse events. The AEs were broken into System Organ Classes (SOCs) with each SOC further divided into individual conditions observed in the field. The report described AEs with percentages representing proportions of reports received. Any percentages should not be taken as incidence rates of occurrence, as this observational data was not a clinical trial. Nonetheless, it should have been evident to Pfizer that its product harmed patients, which included permanent harms and 1,223 deaths.
Within the first three months after rollout of product, providers in the field reported damages across all organ systems to Pfizer. Reference the table below. This table includes special concern areas being tracked by Pfizer through 2020 and 2021. The first special concern, anaphylaxis, is considered an “Identified Risk” (IR), Vaccine-Associated Enhanced Disease (VAED) is considered a “Potential Risk” (PR). The third category of “Missing Information” (MI) concerns “Pregnancy and Lactation,” “Use in Pediatric Individuals,” and “Vaccine Effectiveness.” These IR, PR, and MI categories were predetermined categories of interest from the EUA 2020 that garnered more information in 5.3.6. All other SOCs charted below fell outside those original categories.
| SOC | Page | Number,
% |
Serious
(N, [%]) |
Non-Serious
(N, [%]) |
Report Author’s Notations |
| Anaphylaxis (IR) | 10 | 2,958
7.0% |
2,341
5.6% |
617
1.5% |
|
| VAED (PR) | 11 | – | – | – | 75 potential cases |
| Pregnancy and Lactation (MI) | 12-13 | 413
0.98% |
84
0.2% |
329
0.78% |
Spontaneous abortions and neonatal deaths reported; alterations to breastfeeding |
| Pediatric (MI) | 13 | 34
0.08% |
24
0.05% |
10
0.02% |
One Facial Paralysis |
| Vaccine Effectiveness (MI) | 13-15 | 1,665*
4.0% |
1625
3.9% |
21
0.05% |
“Serious” is considered a case of COVID; no immunity conferred |
| Cardiovascular | 16 | 1,403*
3.3% |
946
2.2% |
495
1.2% |
130 myocardial infarctions, 91 cardiac failures |
| COVID-19 | 17 | 3,067*
7.4% |
2,585
6.1% |
774
1.8% |
Unremarkable; deals with positive cases |
| Dermatological | 17 | 20
0.05% |
16
0.04% |
4
0.01% |
Unremarkable; Reactions |
| Haematological | 18 | 932*
2.2% |
681
1.6% |
399
0.95% |
Numerous examples of spontaneous bleeding from mucous membranes |
| Hepatic | 18-19 | 70*
0.2% |
53
0.13% |
41
0.1% |
Metabolic alterations within the liver |
| Facial Paralysis | 19-20 | 449*
1.07% |
399
0.95% |
54
0.12% |
Authors refer to studies C4591001, C4591011, C4591012, C4591021 |
| Immune-Mediated and Autoimmune | 20 | 1,050*
2.5% |
780
1.9% |
297
0.70% |
32 Pericarditis, 25 Myocarditis |
| Musculoskeletal | 20-21 | 3,600*
8.5% |
1,614
3.8% |
2,026
4.8% |
3,525 Arthralgia |
| Neurological | 21 | 501*
1.2% |
515
1.2% |
27
0.06% |
204 Seizure, 83 Epilepsy |
| Other | 21-22 | 8,152*
19.4% |
3,674
8.7% |
4,568
10.8% |
7,666 Pyrexia
Herpetic conditions |
| Pregnancy Related | 22 | – | – | – | Refers to pages 12-13 |
| Renal | 22 | 69*
0.17% |
70
0.17% |
0
0% |
All serious: 40 acute kidney injury, 30 renal failure |
| Respiratory | 22-23 | 130*
0.3% |
126
0.3% |
11
0.03% |
44 respiratory failures |
| Thromboembolic Events | 23 | 151*
0.3% |
165
0.4% |
3
0.007% |
60 Pulmonary Embolism, 39 Thrombosis, 35 Deep Vein Thrombosis (DVT) |
| Stroke | 23-24 | 275*
0.6% |
300
0.7% |
0
0% |
All serious; Ischaemic and Haemorrhagic conditions reported |
| Vasculitis | 24 | 32*
0.08% |
25
0.06% |
9
0.02% |
Specific condition leading to one fatality not noted |
| Medication Error | 26 | 2056*
4.9% |
124
0.29% |
1932
4.6% |
Seven fatalities not categorized as “Serious.” Authors lack information leading to fatalities, considered noncontributory |
(N, [%]): Annotation refers to number of cases (N) and the proportion of AE reports [%]
*: denotes counting discrepancies within the 5.3.6 report
Report Author’s Annotations: Any commentary in this column represents sample highlights from each SOC. All readers are encouraged to read the 5.3.6 document to understand the scale, depth, and width of Pfizer’s aggregated safety reports from the field.
Accounting was not well-done in this Pfizer report and was best illustrated by Table 1 (5.3.6, p.7). The authors reported the adverse events by age brackets that were not standardized in age range, which led to potential issues in understanding age-related effects. The age groupings were <17, 18-30, 31-50, 51-64, and >75. This non-standardized approach obscured any age-related effects among AEs. Most AEs occurred in the 31-50 range, but this age range was also the widest age range. When this document first became available for review, it was difficult to make sense of how data was gathered and grouped. More information on this topic emerged later in the PV document. Table 1 did relay important findings. There were 1,223 deaths in the field that providers thought were product related. There were also 520 reports of AEs with sequelae, 11,361 reports of “not recovered at the time of report,” and another 9,400 events without known resolution criteria.
There was one concept Pfizer confirmed in their reporting system regarding latency. When aggregated, it was apparent that reported AEs developed immediately after product use. The median latency for each category is less than a week. See the table below. By Pfizer’s own standard from the EUA 2020 (“From a safety perspective, a 2-month median follow-up following completion of the full vaccination regimen will allow identification of potential adverse events that were not apparent in the immediate post-vaccination period. Adverse events considered plausibly linked to the vaccination generally start within 6 weeks of vaccine receipt”), this realization alone should have been enough to suggest AEs were product related. Yet very consistently and predictably throughout the 5.3.6 report, Pfizer stated, “Conclusion: This cumulative case review does not raise new safety issues. Surveillance will continue.” It begs the question when Pfizer would admit there were significant safety issues with its product and when they would notify the public.
| SOC | AE Development Range | AE Development Median |
| Cardiovascular | <24 hours – 21 days | <24 hours |
| Covid-19 | <24 hours – 374 days | 5 days |
| Dermatological | <24 hours – 17 days | 3 days |
| Haematological | <24 hours – 33 days | 1 day |
| Hepatic | <24 hours – 20 days | 3 days |
| Facial Paralysis | <24 hours – 46 days | 2 days |
| Immune-Mediated and Autoimmune | <24 hours – 30 days | <24 hours |
| Musculoskeletal | <24 hours – 32 days | 1 day |
| Neurological | <24 hours – 48 days | 1 day |
| Other | <24 hours – 61 days | 1 day |
| Renal | <24 hours – 15 days | 4 days |
| Respiratory | <24 hours – 18 days | 1 day |
| Thromboembolic | <24 hours – 28 days | 4 days |
| Stroke | <24 hours – 41 days | 2 days |
| Vasculitic | <24 hours – 19 days | 3 days |
5.3.6 – Conclusion Summary Statement
The 5.3.6 document was reviewed elsewhere in the War Room/DailyClout Pfizer Documents Analysis Project, because it was dense and required further exploration as a result. In the context of what Pfizer knew about safety and efficacy in March 2021 and remembering 5.3.6 was not available to the public without a court order, Pfizer confirmed its product caused significant, severe AEs across all organ systems. What could have been chance AEs in the EUA 2020 C4591001 study were substantiated by field reporting. There were many more AEs than MI, CV, appendicitis, and Bell’s palsy. Death was confirmed as an adverse event based on field reports. Per Pfizer’s EUA 2020, any findings within six weeks would reasonably have been linked to the product. These AEs were often reported within days of product administration. By March 2021, Pfizer knew its product had safety issues, and it knew from the EUA that its efficacy was questionable at best, and invalid or null at worst.
Pharmacovigilance Plan for Biologic License Application (PV)
Report Date: 28 July 2021
Obtained by Court Order
The PV document updated and tracked Pfizer’s plans to detect and to address safety signals. The 99-page document summarized studies and findings up to the date it was published. It added myocarditis and pericarditis as concerning adverse events (AEs) related to the product. Other System Organ Classes’ (SOCs) AEs were on the same scale as pericarditis and myocarditis, yet they were ignored as important risks. After the EUA 2020, Pfizer should have been curious about C4591001 AEs, specifically MI, CV, and facial paralysis (Bell’s Palsy). In 5.3.6 reporting, it identified 130 MI, 275 strokes, and 449 paralyses among many other AEs compared to just 32 cases of pericarditis and 25 cases of myocarditis. There were 165 serious thrombolytic events reported as a separate category in 5.3.6 as well. No AEs were addressed from 5.3.6 other than the predetermined list from the EUA 2020 (IR, PR, MI), and the newly added cardiac AEs (listed under “Immune-Mediated/Autoimmune” on p. 20 in 5.3.6). PV does not provide updated data on MI, CV, paralyses, or thrombolytic events. For reference, appendicitis does not even appear in 5.3.6. What was once witnessed and discussed in the EUA 2020 C4591001 clinical trial and witnessed in field reporting received no further mention in PV. No warnings reached the public on potential harms. Claims of efficacy remained high, and no additional safety signals were addressed from other SOCs.
PV identified ongoing studies that may develop knowledge on efficacy and safety. When a search for those studies was completed on clinicaltrials.gov, many studies did not appear (last checked May 22, 2023). C4591001 was listed as completed on February 10, 2023. No results are available. C4591015, a clinical trial focused on pregnant women, was completed on July 15, 2022. It listed “Primary Endpoints” as 4-30-2023. No results are available. BNT-162-01 showed the results were submitted for review on April 11, 2023. No results are available. C4591007 was listed as pending completion on October 3, 2023. The following clinical trials were listed in PV and were not found on clinicaltrials.gov: C4591008, C4591009, C4591011, C4591012, C4591022, W1235284, and W1255886. PV listed pending report dates for many of these studies. No interim results appear online, as many studies likewise do not appear. Notes on these studies appear in Appendix 1 of this report.
The most important pages of the PV report dealt with vaccinations to the placebo cohort in the EUA study, C4591001. In the EUA 2020, Pfizer outlined the statistical evaluation problems if it vaccinated more than 20% of the placebo cohort (EUA 2020, p. 53). Table 5, “Exposure to BNT162b2 by Age Group and Dose (C4591001) – Open Label Follow-up Period – Subjects Who Originally Received Placebo and Then Received BNT162b2 After Unblinding,” showed Pfizer vaccinated 19,696 placebo participants, representing the entirety of their placebo cohort, by March 12, 2021 (PV, p. 18-19). Pfizer continued to cite the C4591001 study throughout PV as an ongoing clinical trial although Pfizer knew the study was no longer valid per its own standards as laid out in the EUA 2020 (p. 53).
Pharmacovigilance Regarding Safety
Pfizer’s acknowledgement of myocarditis and pericarditis set a precedent for what AEs Pfizer took seriously as safety signals. Yet, Pfizer ignored other AEs. Reference the chart below to compare other SOCs from 5.3.6 against myocarditis and pericarditis as reported in PV. Hundreds of serious AE reports occurred across all SOCs including fatalities and unresolved conditions. There were just 32 cases of pericarditis and 25 cases of myocarditis in 5.3.6. All other SOCs exceeded myocarditis and pericarditis in 5.3.6 and are not mentioned in PV. Other AEs were on scale with myocarditis and pericarditis yet were not added as publicly acknowledged AEs for informed consent. Pfizer seemingly broke from its own standard by ignoring other significant product harms that it observed at the degree as accepted harms.
Pfizer does acknowledge a serious risk pattern from its product through additional product doses. “Evaluation by the US CDC has found reports [of myocarditis and pericarditis] to be most frequent in adolescent and young adult male patients following the second dose of vaccine” (Bold added. PV, p. 50). The appendix of the EUA 5-11 noted the emergence of AEs after additional doses as acknowledgement of a dose-response effect (EUA 5-11, p. 46). The EUA 2020 acknowledged higher rates of AEs after dose two and also noted more AEs in younger participants (EUA 2020, p. 6, p. 42, and p. 56). Pfizer understood there was a relationship between AEs and continued product exposures, and it was observed across the documents. This example with myocarditis and pericarditis was the only place Pfizer admitted the connection between additional doses and the risks of significant AEs. Within the context of the serious AEs across all organ systems, it is reasonable to assume additional doses increase the risks of other types of AEs. This assumption would require a mechanism to explain how the product damaged all organ systems as opposed to narrower, specific types of damage.
| System Organ Class | Document | Serious | Fatal | Unresolved |
| Myocarditis (added) | PV | 459 | 14 | 106 |
| Pericarditis (added) | PV | 370 | 3 | 63 |
| Cardiovascular | 5.3.6 | 946 | 136 | 140 |
| Haematological | 5.3.6 | 681 | 34 | 267 |
| Hepatic | 5.3.6 | 53 | 5 | 14 |
| Facial Paralysis | 5.3.6 | 399 | 0 | 183 |
| Immune-mediated or Autoimmune *** | 5.3.6 | 780 | 12 | 215 |
| Musculoskeletal | 5.3.6 | 1,614 | 0 | 959 |
| Neurological | 5.3.6 | 515 | 16 | 89 |
| Other | 5.3.6 | 3,674 | 96 | 1,429 |
| Renal | 5.3.6 | 70 | 23 | 15 |
| Respiratory | 5.3.6 | 126 | 41 | 18 |
| Thromboembolic | 5.3.6 | 165 | 18 | 49 |
| Stroke | 5.3.6 | 300 | 61 | 85 |
| Vasculitic | 5.3.6 | 25 | 12 | 1 |
| (IIR) Anaphylaxis | PV | 2341 | 9 | 229 |
| (IPR) VAED | PV | 138 | 38 | 65 |
| (MI) Pregnancy | PV | 75 | 38 | – |
| (MI) Lactation | PV | 5 | – | – |
| (MI) Pediatric | PV | 24 | 0 | 16 |
This table demonstrates that AEs from all SOCs are on the same risk scale as the added AEs of myocarditis and pericarditis. Other SOCs from 5.3.6, in fact, exceed them.
(added) AEs now included as safety signals. The occurrences are not from 5.3.6.
*** This category from 5.3.6 contained results for myocarditis and pericarditis.
(IIR) Important Identified Risk – considered an important safety signal.
(IPR) Important Potential Risk – considered a potential safety signal.
(MI) Missing Information Category
Pfizer delivers on a possible mechanism through its discussion on lab-derived efficacy measures, where the company acknowledged it knew about systemic spread of the product. Pfizer knew from rat studies (pp. 9-10) that the product ingredients did travel away from the injection site and aggregated elsewhere (liver, spleen, adrenal glands, ovaries). Pfizer reassured the public that fertility was not affected, and the company touted immunity in offspring, too (PV, p. 11). Nonetheless, this important piece served as a mechanism for breadth of AEs witnessed in its documents. Pfizer may not have had a singular type of AE in large excess, but it witnessed and documented a variety of AEs across SOCs. Pfizer’s documentation of systemic spread should have allowed them to connect its product to harms. Harms occurred in any organ system exposed to Pfizer’s product, and harms occurred with additional exposures to the product.
For reference before the EUA 5-11, Pfizer did review animal studies and introduced lab values in animal models to determine efficacy. Investigators claimed 100% efficacy in immune response in Rhesus Macaques based on chemical immune reaction (PV, p. 9). Although provocative, this reaction would not necessarily indicate human immunity to COVID. Although not evident in this time frame, Pfizer’s celebration of 100% efficacy based on lab titers in animals served as the preamble to using lab-based titers as a substitute for clinical trial data. The upcoming EUA 5-11 expanded this concept of replacing clinical trial data Pfizer presumably knew were not valid.
A discrepancy noted in 5.3.6 received some clarifying information in PV. The age brackets for AE reporting were unusual in 5.3.6 with non-standardized intervals. There was a large age bracket of ages 31 to 50, while other brackets covered about 10 years or less. When authors shared statistics from their safety database, notable coincidences emerged. Myocarditis in ages over 16 occurred most often in young men with a mean age of 37.2 years old and a median age of 32.0 years old (PV, p. 48). For pericarditis in ages over 16, there was no gender difference, and the mean age was 51.5 years old, while the median age was 51.0 years old. The way ages were assembled in 5.3.6, split and diluted myocarditis AEs. In the upcoming EUA 5-11, it was shown again that myocarditis occurred most often in males under age 40 with no incidence rate provided by the investigators (EUA 5-11, pp. 14-15). Investigators did provide incidence rates for these AEs for patients between the ages of 12 and 17. It was striking how Pfizer reported these demographics across documents and how it grouped these cardiac conditions under a different category in 5.3.6. It hinted at something specific with myocarditis in men ages 18 to 39, but there was never an explanation about it. Elaboration by Pfizer investigators would be helpful for understanding how they chose to report these findings and if there were important findings in this age group. With investigators speculating about subclinical, long-term damages in EUA 5-11 (p. 15), and through documentation of various severe AEs leading to death, Pfizer should share what it knows about this avoided age group.
Pharmacovigilance Plans
Section III (PV, pp. 71-92) dealt with the actual Pharmacovigilance plan. This section outlined the courses for current and future studies. Pfizer reviewed the categories of focus. There were Important Risks (Anaphylaxis, Myocarditis, and Pericarditis), Important Potential Risks (VAED/VAERD), and Missing Information (Pregnancy/Lactation, Vaccine Effectiveness, Use in Pediatrics <12). Pfizer outlined its sources for signal detection on PV pages 71-72, which included references to literature and to Web-based reporting systems. Pfizer documented that it knew what was happening with its product in scientific literature, in the field, and within its own reports. Pfizer planned to perform future studies for each category above. Studies of other SOCs were not planned. Perhaps safety signal detection would take place coincidentally, but Pfizer had already ignored safety signals to date.
Pages 73-84 outlined Pfizer’s intent to complete further studies to evaluate efficacy and safety. Studies were outlined by category with due dates specified. Many interim report dates had passed, without reporting, by May 22, 2023. Clinical trial C4591001 was the first study listed on the list of ongoing studies (PV, p. 92). Pfizer intended to make use of this study despite tampering with the placebo cohort months prior to this Pharmacovigilance plan.
Consider what it meant when the C4591001 clinical trial was not completed to term. The claims of efficacy and safety have never been supported. There were only sparse, preliminary results of efficacy based on statistical misrepresentation. Adverse events indicated the product perhaps was not safe in the EUA 2020 and definitely not safe in 5.3.6 by March 2021. The clinical trial was meant to run to 24 months to allow for a proper and robust evaluation of two large cohorts. Pfizer destroyed this trial before relevant results were ever realized. Whatever remained of the trial was completed on February 10, 2023, but even those results are still not available.
The problems with C4591001 made it even more imperative to complete the other studies listed within the PV document. With that in mind, our team set out to verify the status of these studies nearly two years after they were planned and promised by Pfizer. It turns out many of these studies do not exist. Pfizer seems to have had no intention of pursuing the relevant clinical trial data needed to determine a valid efficacy statement. Its dismissal of safety signals both in its own C4591001 trial and in field reporting suggested the company had no strong interest in product safety signals. The absence of promised studies to determine efficacy and to monitor safety completed its failure of honest evaluations.
PV – Conclusion Summary Statement
By July 2021, Pfizer observed its product traveled throughout the body and caused AEs across all organ systems in immediate timeframes after administration with additional doses increasing the likelihood of harm. It also became apparent Pfizer had no intention to report those observations to the public in those terms. Clinical trials planned and listed within PV were also abandoned. If C4591001 was going well, it would have been reported ad nauseum. Since C4591001 was altered well ahead of this report, Pfizer hoped the introduction of titers would give an alternative measure to claim efficacy regardless of disease protection. Investigators in the EUA 5-11 (p. 17) documented this lab-based evaluation was not valid for proving protection from COVID.
Consider the political environment and mandates at the time of this published report in 2021. Pfizer knew it had these problems, and yet the company allowed public statements on efficacy and safety to continue unopposed. The decision not to halt product use represented a top-to-bottom failure at Pfizer. The people compiling these reports were subject matter experts. They knew what the findings meant even as they reported a lack of safety concerns and as they reported high efficacy. They understood every problem posed so far. Even with what Pfizer learned by the time it published PV, the company continued onward to the children.
Where does this lead in the next EUA for five- to 11-year-old children in October 2021? Read this section understanding that the interim results for the young 12- to 15-year-old cohort are due within weeks. There appears to be a rush to complete the EUA 5-11 before relevant trial information becomes available.
Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum
Submission and Receipt Date: October 6, 2021
Review Completion Date: October 29, 2021
After nearly a year of product use and with investigators knowing the issues with safety and efficacy, one would hope for the EUA for five- to 11-year-olds, the EUA meant to authorize use for the youngest Americans, to lay out a very logical case for product use. This document should have been Pfizer’s best effort, but it was not. The document itself appeared hastily constructed suggesting several authors assembled it quickly with disjointed opinions. It contained typos, incoherent commentary, and contradictory narratives. These narratives included claims that vaccinating children would stop spread, although investigators provided no evidence to support the claim and subsequently listed the claim itself as a gap in their knowledge. Investigators also attempted to suggest titers could represent efficacy and later suggested it was not a valid measure. Another narrative included the conclusion of a favorable risk-benefit ratio and yet showed an unfavorable risk-benefit ratio while admitting the COVID risk to children was always minimal.
The primary conclusions made by investigators in the EUA 5-11 were, again, based on weak evidence. Authors concluded efficacy using small numbers and lab values. They did not draw substantial support from C4591001. Authors concluded safety in the face of mounting evidence that the product was not safe. They consistently concluded beneficial risk-benefit ratios while demonstrating with computer modeling that they had an unfavorable risk-benefit ratio. Tucked into the appendix is an admission that investigators understood a dose-response problem with the product (EUA 5-11, p. 46). They learned in C4591007 that AEs were related to both dosage and dose number. Investigators speculated about what these findings could mean for long-term safety (EUA 5-11, p. 15).
EUA 5-11 Regarding Efficacy
In the clinical trial C4591001, investigators used weak evidence for efficacy. In EUA 5-11 (using study C4591007), they relied on a similar format. After two months of follow-up, they noted three COVID cases (out of 1,518 participants) in the vaccine group compared to sixteen cases (out of 750 participants) in their placebo group (p. 26). The incidence rate was 0.02% in the vaccine group and 2.13% in the control group. These percentages are statistically significant but, again, take place over a very short time span. Efficacy is not well-supported by this evidence.
Curiously, in the eleven months since the original EUA 2020, investigators did not report great increases in follow-up in C4591001. They reported around 60% of test and placebo cohorts at four or more months of follow-up, leaving around 40% of the cohorts at much less follow-up (EUA 5-11, p. 12). Pfizer cut off data collection on March 12, 2021, leaving a six-month gap before the EUA 5-11. The data cutoff is consistent with Pfizer’s understanding that the clinical trial effectively ended after vaccination of the entire placebo cohort. Efficacy claims in the October 2021 EUA for five- to 11-year-olds lack support from the original trial as a result. With the added context from PV (pp. 18-19) which was not made available to the public until after the court order, the public can now see that Pfizer abandoned its efficacy monitoring in C4591001. Pfizer, per their own standard (EUA 2020, p. 53), knew its efficacy analysis was no longer valid without a placebo cohort and terminated its data collection on March 12, 2021. If Pfizer had continued the clinical trial with blinded placebos as planned, it would have had up to six more months of data for EUA 5-11. Instead, Pfizer’s investigators turned to vaccinating children knowing they destroyed what could have been the most important data to parents. The public was denied whatever truth C4591001 could have provided. The public once again was forced to accept another document lacking evidence.
The investigators understood the problems with short-term follow-up of only two months. They introduced immunobridging as a metric for efficacy. In brief, investigators used bloodwork to look for production of antibodies as a response to product use. They assumed an antibody titer implied protection. On page 17 (EUA 5-11), investigators made it clear that “the immune marker(s) used for immunobridging do not need to be scientifically established to predict protection,” yet they used immunobridging to determine efficacy. Investigators claim 100% efficacy (EUA 5-11, p. 13) based on these titers despite a subsequent admission on page 17 that they do not know what titer concentration would confer protection. Investigators used a test for efficacy that they knew was not valid.
EUA 5-11 Regarding Safety
Pfizer identified a dose-response relationship and connected it to the potential for long-term damages. The EUA Appendix (p. 46) discussed the dosage reduction in children. Investigators found, during C4591007, two factors that led to more adverse reactions: 1) the dose number, and 2) the dosage. Investigators found a dose-response relationship between the product and AEs in their own trial. Furthermore, the number of doses being related to adverse events was significant because it suggested cumulative risks with continued dosages. Investigators did not report severe adverse events in the appendix like myocarditis. The solicited AEs for which they were checking became more severe. Nonetheless, these dose-dependent concepts dovetailed with potential long-term concerns that investigators had about the product (EUA 5-11, p.15). The investigators suggested that subclinical damages would aggregate over time through repeated doses and AEs would eventually manifest clinically in children. With negligible risk to children from COVID, AEs from product use posed more risk than the disease itself.
There was an explanation for the addition of pericarditis and myocarditis in this EUA that was not present in PV (EUA 5-11, p. 13). There were two cases of pericarditis in the C4591001 study by the June 2021 cutoff date. One case was a 55-year-old male 28 days (“within 6 weeks,” a standard from EUA 2020) after dose #2 (risk factor “dose number” in EUA 2020, p. 6; PV, p. 50; EUA 5-11, p. 46). Investigators deemed this adverse event unrelated to product in both PV and EUA 5-11 despite the factors identified by investigators that would suggest a relationship. The second case took place in an unblinded placebo, a male 16 years of age (risk factor “young male” in PV, p.50) that developed myopericarditis two days after dose #2. After two months of symptoms, his cardiologist still recommended “limited activity” (EUA 5-11, p.13). PV, in July 2021, denied product involvement even when faced with a known AE related to product use: “Two (2) serious adverse events [PT Pericarditis] were reported, both deemed not related to study treatment by the Investigator” (PV, p.47). An admission that the AE was related to Pfizer’s product finally emerged within the October 2021 EUA 5-11. The product resulted in an unresolved condition at the last follow-up. In this case, “The investigator concluded that the there [sic] was a reasonable possibility that the myopericarditis was related to vaccine administration due to the plausible temporal relationship. FDA agrees with this assessment” (EUA 5-11, p. 13).
Investigators attempted to explain away a known AE risk in PV, got caught, and were forced to amend the report for the EUA 5-11. There was a discrepancy here. The structure of these documents suggested this 16-year-old patient’s side effect was important to product risk labeling. Yet, when he was identified in the PV document as unrelated, myocarditis and pericarditis were already identified as important risk factors. It begets the question whether critical evaluation was taking place. Further information from the investigators is needed as this issue is not explained clearly in the provided documents.
Investigators should have been suspicious of product involvement with AEs per their own standard from EUA 2020, yet they continued the denial of product involvement with AEs through 5.3.6 and PV despite relevant factors learned along the way. Only in EUA 5-11 did they finally admit the product could have been related to the 16-year-old’s AE. They never admitted the potential for product involvement in the 55-year-old male’s AE despite relevant factors involved that they identified.
Pericarditis and myocarditis were added as label warnings based on this one case above from C4591001 and based upon VAERS reports (EUA 5-11, pp. 13-14). (PV notes “Important Identified Risk ‘Myocarditis and Pericarditis’” on page 8 sourced from Pfizer Safety Database). Investigators finally acknowledged the risk of myocarditis and pericarditis from product use by the October 2021 in EUA 5-11. What finally forced this acknowledgement? Was it because the side effects took place in young males and would be more difficult to explain away than other side effects? A thorough explanation from investigators is required to eliminate this suspicion, especially after the age bracket issues identified in 5.3.6 with young patients ages 18-39.
Myocarditis and pericarditis adverse events were on scale with other AEs reported by the field in 5.3.6, yet Pfizer ignored or dismissed those additional AEs. “Review of passive surveillance AE reports and the Sponsor’s periodic safety reports did not indicate any new safety concerns.” They continue digging, “ No unusual frequency, clusters, or other trends for AEs were identified that would suggest a new safety concern, including among the reports described as involving children 5-11 years of age” (EUA 5-11, p. 14).
The EUA investigators posed a serious set of facts revolving around pericarditis and myocarditis. The Food and Drug Administration (FDA) analysis from Optum healthcare claims database estimated incident rates in ages 16 to 17 of 200 cases per million (0.02%) and in 12- to 15-year-old of 180 cases per million (0.018%) (EUA 5-11, p.15). These rates of adverse events occurred at a similar rate as the AEs of MI, CV, appendicitis, and Bell’s palsy in EUA 2020 (pp. 37, 40). Investigators suspected that the damage was more significant than the rates above: “Information is not yet available about potential long-term sequelae and outcomes in affected individuals, or whether the vaccine might be associated initially with subclinical myocarditis (and if so, what are the long-term sequelae).” (Bold Added, EUA 5-11, p. 15). This statement was the first time among documents reviewed that the authors turned to long-term questions of adverse events. Investigators went further: “A mechanism of action by which the vaccine could cause myocarditis and pericarditis has not been established.” This unknown mechanism should have been a serious concern overall in light of the variety of AEs observed and in light of animal studies showing the spread of product throughout the body. Pfizer may not have known the exact cellular mechanism linking its product to AEs. However, the company should have been able to piece together that systemic spread of product caused damage across all organ systems in a dose-response relationship in at least the short term and potentially also in the long term. It suspected subclinical damages would affect patients on a significant delay. What is yet to be learned about males ages 18-39? The compilation of this set of safety concerns should have been a full-stop event for Pfizer. The constellation of evidence indicated Pfizer knew it did not have a favorable risk-benefit ratio as investigators identified significant product issues that would cause more damage than the disease itself.
EUA 5-11 – Risk-Benefit Analysis
Investigators are honest regarding the minimal risks of COVID to the 5-11 age group. Authors note on page 7 (EUA 5-11) the reality that 15% to 50% of patients are asymptomatic even when they have COVID. They recover within one to two weeks and have milder symptoms than adults. By the time EUA 5-11 was published, there were 44 million identified cases of COVID in the United States with 722,000 deaths (EUA 5-11, p.7). About 8.7% (3.8 million) of cases were in the 5 to 11 age group. A rational assumption was that many more asymptomatic cases were never diagnosed and did not factor in the rates of AEs from COVID. Among the millions of known COVID cases, there were 4,300 hospitalizations and 146 deaths total included in the EUA 5-11 data. The risk of hospitalization and/or death was negligible for the 5-to-11 age group.
These statistics did not support vaccination in this cohort outright because the risk was nearly zero. The benefits would have been imperceptible as so few young children were affected by significant disease. Even a vaccine with rare risks posed as much risk or more risk than the disease itself. Here was what the authors wrote on page 37 (EUA 5-11): “While no cases of severe COVID-19 were accrued during study follow-up to date, it is highly likely that vaccine effectiveness against severe COVID-19 among children 5-11 years of age will be even higher than vaccine effectiveness against non-severe COVID-19, as is the case in adults.” (Bold Added.) This conclusion was incoherent. The data set for C4591007 cannot support this claim since there were no severe disease occurrences (EUA 5-11, p. 26). It was a hopeful speculation. Investigators doubled down on page 38 (EUA 5-11), noting that “widespread deployment” will “have substantial effect on COVID-19 associated morbidity and mortality in this age group [5-11 years].” Their lab values did not support this claim per their own words (p. 17, EUA 5-11). Their own statistics on epidemiology refuted this statement, too. “Widespread” cannot be applied to events that rarely occur. They shared no data from C4591007 in this EUA related to transmission. Their conclusion was wrong because it was unsubstantiated in every respect.
Investigators clearly understood that COVID-19 was tolerated well in the young, and they would have understood that reality was a barrier to product deployment. Their solution was to discuss disease transmission as a new concept in EUA 5-11 (p. 8). Transmission was not discussed in the original EUA in 2020, 5.3.6, or the PV document, yet it emerged in this document. By page 9 (EUA 5-11), they argued dangers posed to adults by transmission from children. Ironically, adults were already approved and could have this allegedly highly efficacious product. Transmission from children should be of no concern to vaccinated adults if Pfizer showed the product works. Investigators went a step further to blame transmission of virus on individuals who are not vaccinated. Again, if the product works, there is limited risk to the vaccinated from the unvaccinated. Pfizer did not provide evidence from C4591007 that the product halted transmission or that unvaccinated individuals caused more transmission. Nonetheless, investigators created an argument that tried to have it both ways. The product supposedly worked well enough to have high levels of protection for adults yet did not work well enough to offer protection around children.
On page 38 (EUA 5-11), investigators documented important “Data Gaps.” Investigators listed “Vaccine effectiveness against asymptomatic infection” and “Vaccine effectiveness against transmission of SARS-CoV-2” as gaps in their knowledge. The investigators, after a section where they argued the need for widespread vaccination in children and declared their product could greatly reduce symptoms and greatly reduce transmission, listed their own conclusions as gaps in their knowledge (EUA 5-11, p. 38). This section highlighted Pfizer’s use of hopeful speculation over data to push for product approval. There can be room to speculate about potential benefits in scholarly work, but the investigators had no data to support their speculations. They had a very limited efficacy statistic from C4591007 and lab titers that they knew did not equate to disease protection. The investigators attempted to jump from two weak data points into a full-throated claim that the product would substantially reduce morbidity, mortality, and transmission. Even under the assumption the product did those things, the investigators never showed that it achieved any of those goals.
The above gaps in benefits were then overlaid with the risks posed to children. On page 38 (EUA 5-11), “…the risk of vaccine associated myocarditis/pericarditis among children 5-11 years of age is unknown at this time.” The investigators’ statement was technically true, but they could have estimated a risk of 0.02% based on myocarditis risks in ages 12-17 (EUA 5-11, p. 15). Based on this statement and the gaps in benefits, the investigators could not have made objective claims that there was a favorable risk-benefit ratio. They admitted openly that they did not know the benefits or the risks. Investigators wanted the public to believe a disease with limited risk to children (4,300 total hospitalizations and 146 total deaths reported in EUA 5-11) justified the widespread use of a product with unsubstantiated efficacy and with safety concerns that they would have known rivaled or exceeded the damage of the disease itself.
After investigators argued a case that should have denied the product approval, investigators turned to computer modeling and showed it definitely should not have been approved. Per the investigators (EUA 5-11, p. 46), for one million vaccinated children during a six-month period, the product would prevent an estimated 45,000 (4.5%) cases, reduce 200 hospitalizations (0.02%), reduce 60 to 80 ICU stays (0.0006%), and prevent zero or one death (0-0.0001%). After vaccinating one million children, a vast majority would have received no benefit. The model factored in risk of myocarditis. Investigators expected about 100 cases of myocarditis (0.01%), about 100 hospitalizations (0.01%), about 30 ICU admissions (0.003%), and zero deaths. The investigators demonstrated in their model extremely limited benefit, in the vicinity of zero percent, and they demonstrated risks on the scale of benefits. Their model did not predict a favorable risk-benefit ratio. It showed it would require tremendous numbers of vaccinations to deter a few COVID hospitalizations. If investigators factored in the numerous other AE risks from 5.3.6, this risk-benefit assessment would have rapidly degraded into the inevitable conclusion that the product risks outweighed any negligible benefits.
EUA 5-11 – Conclusion Summary Statement
By the completion of the EUA 5-11, investigators still had efficacy shortcomings. Nearly a year into product use, the public should have heard about the successes of the C4591001 study in motion, yet that was not the case. Unbeknownst to the public, C4591001 was effectively destroyed by Pfizer, negating the ability to derive long-term data. The statistics from C4591007 were likewise weak. Investigators began discussions on boosters, another sign they had weak or absent efficacy. Investigators showed higher doses and cumulative doses contributed to adverse events yet refused to acknowledge risks accumulated in EUA 2020, 5.3.6, and PV. They concluded a favorable risk-benefit ratio yet demonstrated it was unfavorable. Investigators introduced transmission as a reason to vaccinate and blamed unvaccinated individuals for transmission. They had no evidence from C4591007 to support either conclusion.
Questions That Need Answers
- What are the results from C4591001 and other ongoing trials?
- What process determined which adverse events were considered legitimate and which adverse events were not? The standard was not clear in Pfizer’s documents. There were inconsistencies in the standards that require explanation by the investigators. The investigators are confident the product is safe. Do ongoing clinical trials support safety? Are field reports in conflict with the clinical trials? If so, reconciliation by investigators is needed.
- The criticisms in this report could be dispelled by strong efficacy and safety measures in the clinical trials. What caused Pfizer to destroy its own clinical trial, C4591001?
- Why did transmission enter in the EUA 5-11 when it was not discussed previously? Was it meant to make the case to vaccinate a population that did not have a practical benefit?
- What did Pfizer know about the profile of adverse events in males ages 18 to 39?
Commentary on the Advisory Committees and the EUAs
In pharmacovigilance, an important step before approval of a new drug is the advisory committee review process. According to the Centers for Disease Control and Prevention (CDC), “Safety is a Priority During Vaccine Development and Approval. Before vaccines are licensed by the FDA, they are tested extensively in the laboratory and with human subjects to ensure their safety” (https://www.cdc.gov/vaccinesafety/ensuringsafety/history/index.html). The Advisory Committee on Immunization Practices (ACIP) is the CDC’s advisory committee recommending vaccines. VRBPAC is the FDA’s vaccine/biologic products advisory board and is part of the Center for Biologics Evaluation and Research (CBER). VRBPAC “…reviews and evaluates data concerning the safety, effectiveness, and appropriate use of vaccines and related biological products which are intended for use in the prevention, treatment, or diagnosis of human diseases…” (https://www.fda.gov/advisory-committees/blood-vaccines-and-other-biologics/vaccines-and-related-biological-products-advisory-committee) These committees effectively had two chances to address product issues before the FDA EUA-approved and the CDC publicly recommended Pfizer’s mRNA COVID vaccine. It does not appear that the committees did their due diligence. A report on the failures of pharmacovigilance within this these committees is planned as upcoming work in the larger WarRoom/DailyClout Pfizer Documents Analysis project.
Conclusion
Efficacy of the BNT162b2 mRNA COVID “vaccine” was not demonstrated by Pfizer during 2020 and 2021. If investigators were pleased with results after six weeks, they could have continued every six weeks with interim reports which could have rolled into 5.3.6, PV, and EUA 5-11.
Pfizer’s declination to continue its own clinical trial by vaccinating placebo participants is a significant problem. There is not an intact clinical trial to show high drug efficacy over time. Maybe there is a good explanation? If so, Pfizer needs to share it, especially with C4591001 ruined and many other studies terminated. Is it possible Pfizer recognized its trial was going to produce unfavorable results and ended it before those results became more obvious? Pfizer would be unable to defend itself using C4591001, especially because it negated the clinical trial by vaccinating the entire placebo cohort in March 2021. The lack of interim trial results, the destruction of C4591001, the shift to antibody titers to try to prove effectiveness, and the addition of hopeful speculation in clinical trial documents indicate problems with BNT162b2’s efficacy.
Safety was not demonstrated by Pfizer. The Company understood its product spread throughout the body, witnessed AEs across all organ systems, witnessed immediate latency, and witnessed dose-response relationships which also caused investigators to speculate about long-term AEs. None of that indicated safety. Taken together, Pfizer, based on its own written standards and its own reports, should have understood its product had significant risks and limited, if any, benefits.
Appendix 1: Study Due Dates from PV Document
| Study Number | Population | PV Due Dates | Results Posted?
Clinical Trials Notables |
| C4591001 (C) | EUA study | Final: 8-31-2023 | No results available
Completed: 2-10-2023 |
| C4591001 (A) | Ages 12-15 | First Report one-month of two dose: 4-30-2021
Six-Month: 10-31-2021 (report due immediately after EUA 5-11). Two Year: 4-30-2023 |
No results available |
| C4591007 (A) | Ages under 12 | First report with up to one-month post-dose: 9-30-2021
Interims: Six Month: 3-31-2022 Two Year: 9-30-2023 |
No results available
Pending Completion: 10-3-2023
|
| C4591008** | US Healthcare Workers | Interims:
6-30-2021 12-31-2021 6-30-2022 12-31-2022 Final: 12-31-2023 |
Not found on clinicaltrials.gov |
| C4591009** | US population | Interim: 10-31-2023
Final: 10-31-2025 |
Not found on clinicaltrials.gov |
| C4591009** | Ages 5-12 | First Report: 9-30-2021
Six Month: 3-31-2022 Two Year: 9-30-2023 |
Not found on clinicaltrials.gov |
| C4591011** | US Military | Interims:
10-31-2022 6-30-2022 12-31-2022 Final: 12-31-2023 |
Not found on clinicaltrials.gov |
| C4591012** | VA System | Interims:
6-30-2021 12-31-2021 6-30-2022 12-31-2022 Final: 12-31-2023 |
Not found on clinicaltrials.gov |
| C4591014 (R) | Efficacy by Kaiser Permanente | Final submission: 6-30-2023 | No results available
Pending completion: 3-25-2024 |
| C4591015 (C) | Pregnant Women | Primary Endpoints: 4-30-2023 | No results available
Completed 7-15-2022 |
| C4591022 ** | Pregnancy, Infant Outcomes | Interims:
1-31-2022 1-31-2023 1-31-2024 1-31-2025 Final: 12-1-2025 |
Not found on clinicaltrials.gov |
| W1235284 | Comparison to other respiratory diseases | Final submission: 6-30-2023 | Not found on clinicaltrials.gov |
| W1255886 | Lower Respiratory Study | Final submission: 6-30-2023 | Not found on clinicaltrials.gov |
| BNT-162-01 (A) | Cohort 13 | First submission: 9-30-2021 | Results not available.
Results submitted 4-11-2023 for review. |
Legend:
**: When searched on http://clinicaltrails.gov, these studies are “Not Found” and the site redirects to C4591001
(A): Active (R): Recruiting (C): Completed
C4591001 is a composite of 7 different studies listed as (A) or (C)
CSR: Clinical Study Report
Endpoints: The principal outcomes that are measured in a clinical trial.
No comments:
Post a Comment