Response to comment from Jessica Hockett:
As
far as I can tell, Azar needed the WHO PHEIC on Jan. 30, 2020 in order
to issue his Jan 31, 2020 decree. Let me know if you see it differently.
I don’t think Azar needed the WHO PHEIC, for several reasons.
To
the extent that the worldwide, coordinated pandemic preparedness system
is a deception project jointly executed by the US
HHS-DoD-DHS-whole-of-government and the UN-World Health Organization, I
think they have coordinated the drafting and adoption of the legal
instruments each institution relies on as authorization for their
respective actions.
As
a coordinated team interested in maximizing public acceptance of the
necessity for any pandemic preparedness and response at all (or routine
communicable disease control and vaccination programs), and government
and WHO authority to actually carry out pandemic preparedness and
response programs, US-HHS-DoD and WHO have a mutual interest in
projecting the credibility of both institutions/conglomerates, by
referring to each others' acts as providing further support for the
course of action taken by each institution.
So
while I don't think HHS/Azar legally needed the WHO-PHEIC to conduct
his own acts and direct the acts of other officials in the United
States, Azar benefited from the WHO-PHEIC/Director-General's performance
because the WHO performance added weight and apparent credibility to
HHS/Azar's own acts, and the WHO-PHEIC/Director-General's performance
benefited from Azar's US-HHS acts, because Azar's performance added
weight and apparent credibility to the World Health Organization's acts.
Neither
legal framework requires either performer to provide physical evidence
that "something is spreading," or poses a threat to health, nor to
submit any such evidence to any fact-finding, evidentiary-review
tribunal.
Here are the reasons why I don't think Azar needed the WHO-PHEIC.
One reason is that one of the statutes
under whose authority Azar acted also authorizes Defense Secretary and
Secretary of Homeland Security (in addition to HHS Secretary) to make
determinations that emergencies exist.
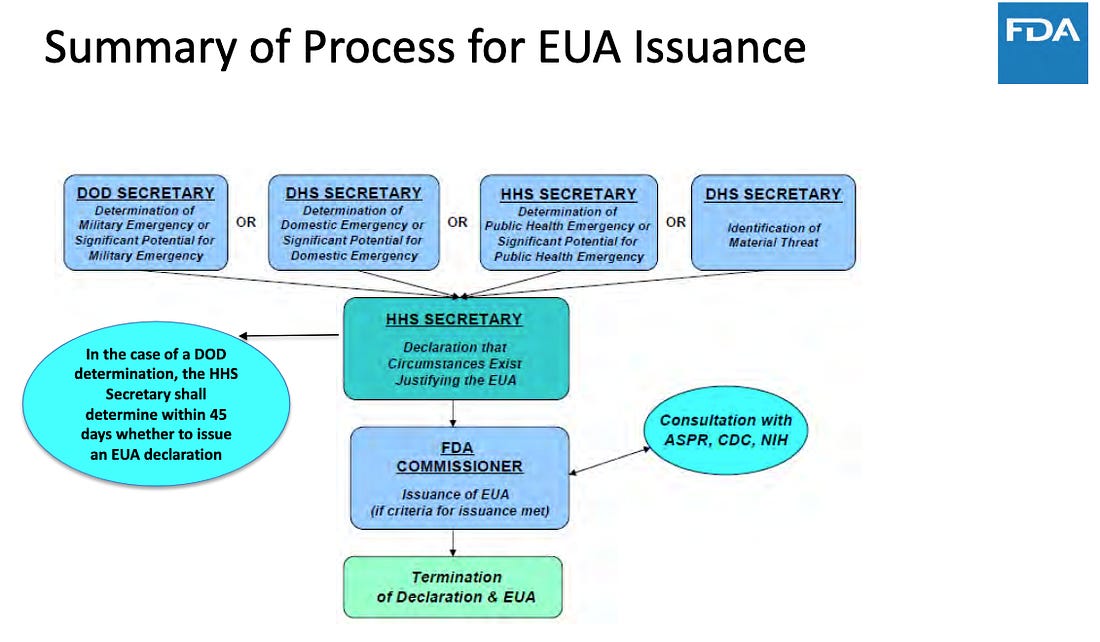
The section of the FDCA actually used by Azar (HHS Secretary) was 21 USC 360bbb-3(b)(1)(C).
21 USC 360bbb-3(b)
“Declaration of emergency or threat justifying emergency authorized use.
(b) Declaration of emergency or threat justifying emergency authorized use
(1) In general. The [HHS] Secretary may make a declaration that the circumstances exist justifying the authorization under this subsection for a product on the basis of—
(A) a determination by the Secretary of Homeland Security that there is a domestic emergency, or a significant potential for a domestic emergency, involving a heightened risk of attack with a biological, chemical, radiological, or nuclear agent or agents;
(B) a determination by the Secretary of Defense that there is a military emergency, or a significant potential for a military emergency,
involving a heightened risk to United States military forces, including
personnel operating under the authority of title 10 or title 50, of
attack with—
(i) a biological, chemical, radiological, or nuclear agent or agents; or
(ii)
an agent or agents that may cause, or are otherwise associated with, an
imminently life-threatening and specific risk to United States military
forces;
(C) a
determination by the [HHS] Secretary that there is a public health
emergency, or a significant potential for a public health emergency, that affects, or has a significant potential to affect,
national security or the health and security of United States citizens
living abroad, and that involves a biological, chemical, radiological,
or nuclear agent or agents, or a disease or condition that may be
attributable to such agent or agents; or
(D) the identification of a material threat
pursuant to section 319F–2 of the Public Health Service Act [42 U.S.C.
247d–6b] sufficient to affect national security or the health and
security of United States citizens living abroad.”
Those
determinations and declarations under Title 21 (Food Drug and Cosmetic
Act) are related to PHE and "material threat" determinations issued
under Title 42 (Public Health Service Act) at 42 USC 247d(a), 42 USC
247d(b)(1), 42 USC 247d-6b(c)(2) and 42 USC 247d-6d(b)(1) [PREP Act
declarations pertaining to the production and use of countermeasures].
42 USC 247d(a)
“Emergencies. If the Secretary determines, after consultation with such public health officials as may be necessary, that-
(1) a disease or disorder presents a public health emergency; or
(2) a public health emergency, including significant outbreaks of infectious diseases or bioterrorist attacks, otherwise exists,
the
Secretary may take such action as may be appropriate to respond to the
public health emergency, including making grants, providing awards for
expenses, and entering into contracts and conducting and supporting
investigations into the cause, treatment, or prevention of a disease or
disorder as described in paragraphs (1) and (2)...”
42 USC 247d(b)(1)
“Public Health Emergency Fund
(1)
In general. There is established in the Treasury a fund to be
designated as the "Public Health Emergency Fund" to be made available to
the Secretary without fiscal year limitation to carry out subsection
(a) only if a public health emergency has been declared
by the Secretary under such subsection or if the Secretary determines
there is the significant potential for a public health emergency,
to allow the Secretary to rapidly respond to the immediate needs
resulting from such public health emergency or potential public health
emergency. The Secretary shall plan for the expedited distribution of
funds to appropriate agencies and entities.”
42 USC 247d-6b(c)(2).
“Material
threat. The Homeland Security Secretary, in consultation with the [HHS]
Secretary and the heads of other agencies as appropriate, shall on an ongoing basis-
(i) assess current and emerging threats of chemical, biological, radiological, and nuclear agents; and
(ii) determine which of such agents present a material threat against the United States population sufficient to affect national security.”
42 USC 247d-6d(b)(1)
“(b) Declaration by Secretary (1) Authority to issue [PREP Act] declaration
Subject to paragraph (2), if
the Secretary makes a determination that a disease or other health
condition or other threat to health constitutes a public health
emergency, or that there is a credible risk that the disease, condition,
or threat may in the future constitute such an emergency, the Secretary may make a declaration, through publication in the Federal Register, recommending, under conditions as the Secretary may specify, the manufacture, testing, development, distribution, administration, or use of one or more covered countermeasures, and stating that subsection (a) [liability protections] is in effect with respect to the activities so recommended.”
Another reason why I don't think Azar legally needed the WHO-PHEIC is that the quarantine regulations
(42 CFR 70 and 71) define “public health emergency” as having five
legal sources, any one of which is legally sufficient, and two of which
are unrelated to WHO acts:
"Public health emergency as used in this part means:
Any
communicable disease event as determined by the [CDC] Director with
either documented or significant potential for regional, national, or
international communicable disease spread or that is highly likely to
cause death or serious illness if not properly controlled; or
Any
communicable disease event described in a declaration by the Secretary
pursuant to 319(a) of the Public Health Service Act (42 U.S.C. 247d(a));
or
Any
communicable disease event the occurrence of which is notified to the
World Health Organization, in accordance with Articles 6 and 7 of the
International Health Regulations, as one that may constitute a Public
Health Emergency of International Concern; or
Any
communicable disease event the occurrence of which is determined by the
Director-General of the World Health Organization, in accordance with
Article 12 of the International Health Regulations, to constitute a
Public Health Emergency of International Concern; or
Any
communicable disease event for which the Director-General of the World
Health Organization, in accordance with Articles 15 or 16 of the
International Health Regulations, has issued temporary or standing
recommendations for purposes of preventing or promptly detecting the
occurrence or reoccurrence of the communicable disease."
Several
state AGs petitioned HHS to remove reasons 3, 4 and 5 from the US
quarantine regulations definitions in July 2022; their petition was
denied by HHS in October 2022. Two of the states then filed a federal
complaint in January 2023; their complaint was dismissed in August 2023.
I
think the most detailed attempt I've made at untangling the
overlapping, mutually-reinforcing relationships between FDCA-authorized
determinations and declarations and PHSA-authorized determinations and
declarations is this post:
Posts about the state AG's challenge to HHS inclusion of WHO acts as among definitions of 'public health emergency:'
Madonna del Popolo. Detail of painting by Federico Barocci.

No comments:
Post a Comment