Herpes Zoster Reactivated Following mRNA COVID Shots, Studies Show
Concerns about the adverse effects of mRNA COVID-19 shots continue to grow a year and a half after they began to be distributed under an Emergency Use Authorization (EUA) granted to Pfizer/BioNTech and Moderna by the U.S. Food and Drug Administration (FDA). Cases of herpes zoster (HZ) following messenger RNA (mRNA) COVID shots have been reported in the U.S. and worldwide.1 2
What is Herpes Zoster?
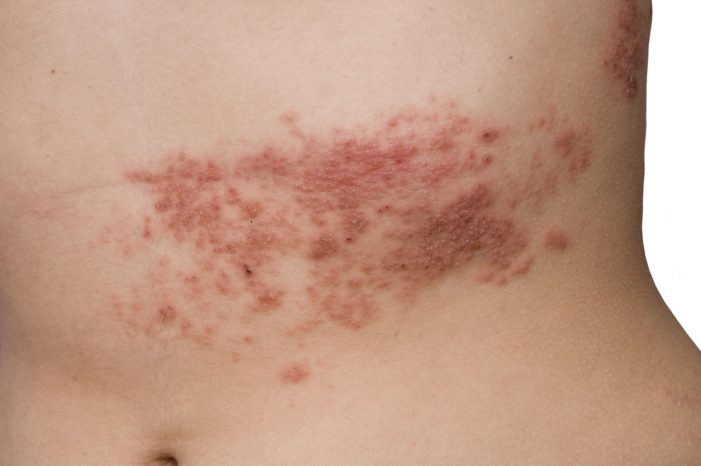
Herpes zoster, also known as shingles, is a viral infection that results in an outbreak of a painful rash or blisters on the skin. The same virus that causes chickenpox (varicella zoster), is known to cause herpes zoster in adults. The virus clinically manifests as rashes or blisters in one area of the body.3 When children contract and recover from a chickenpox infection, the virus remains dormant in the human body and has the potential to reactivate again as herpes zoster in adulthood.4
Recent studies and case studies have shown the reactivation of herpes zoster virus following administration of mRNA) COVID shots, which includes Pfizer/BioNTech’s BNT162b2 (also known as “Comirnaty”) and Moderna’s mRNA-1273 (or “Spikevax”) biologics.5
Herpes Zoster Develops After mRNA Shots in Older and Immune Compromised Patients
A 2022 study published in the British Journal of Clinical Pharmacology is the first epidemiological evidence that suggests an increased risk of HZ after getting mRNA COVID shots.6 The study shows that HZ may occur shortly after mRNA COVID shots at higher frequency than is reported following influenza vaccinations.
The study also shows that the risk of HZ reporting was more common among older age groups than those who are under 40 years old. HZ infections may occur in patients previously infected with varicella zoster virus, especially in patients who are immune compromised and mainly presents as shingles, cranial nerve palsy, meningitis, myelitis, retinitis or hepatitis. The researchers observed that currently HZ reactions following mRNA COVID shots are mild and infrequent.7
In another systemic review published in 2021 in the Journal of Cosmetic Dermatology, a total of 54 HZ cases were investigated, which included 27 male and 27 female patients that were cases with known risk factors for herpes zoster, which included age 50 or above and having chronic disease, such as a malignancy or an immunological, metabolic or psychiatric disorder.8
The average period between development of herpes zoster and administration of COVID shots was approximately seven days. Eighty percent (36/45 cases) of the sample cases developed herpes zoster following the first COVID shot among those who received the mRNA biologic.9
Case Studies of Herpes Zoster in Young, Healthy Adults After COVID Shot
A case series published in 2022 in the Journal of Cutaneous Medicine describes four cases of herpes zoster in young healthy adults with no known risk factors for varicella zoster virus reactivation.10 The first case was a healthy 27-year-old female with polycystic ovarian syndrome (PCOS), who received her second dose of Pfizer/BioNTech’s BNT162b2 biologic in her left arm. Thirty days later, a group of itchy, painful lesions erupted on the right shoulder. She had a history of past varicella zoster infection as a child but no history of previous HZ.11
The second case was a healthy 20-year-old male with a past medical history of asthma, who presented with HZ six days after receiving his second injection of BNT162b2 in his left arm. The lesion appeared in the on the left abdomen. The patient had received the chickenpox vaccine as a child.12
The third case was a healthy 32-year-old female with a history of nephrolithiasis (kidney stones) who presented with HZ 28 days after receiving the second dose of Moderna’s mRNA-1273 biologic in her left arm. The lesion was located over the right breast area. Patient had a history of childhood infection of chickenpox but no history of HZ.13
The fourth case is a healthy 27-year-old female with no past medical history, who presented symptoms of HZ 39 days after receiving a second dose of mRNA-1273 in her right arm. The lesion was located on her left arm and there was a history of chickenpox infection in childhood but no history of prior HZ.14
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:
No comments:
Post a Comment