Monkeypox Cases in U.S Coincide With Vaccine Purchases by BARDA
The World Health Organization (WHO) announced on May 21, 2022 that more than 100 confirmed or suspected cases of monkeypox had been identified in 12 countries, including between one and five confirmed cases in the U.S.1 Most of the cases have occurred in Europe with higher numbers in Portugal, Spain and the U.K. WHO officials stated current evidence suggests “those who are most at risk have had close physical contact with someone with monkeypox, while they are symptomatic.”
The detection of monkeypox cases in Europe and the U.S. is highly unusual because monkeypox has previously been mostly confined to tropical areas of West or Central Africa.2 Monkeypox belongs to the same family of orthopox viruses as smallpox (variola) and the CDC states that smallpox (vaccinia) vaccines can be used to prevent monkeypox.3
On May 18, 2022, biotechnology firm Bavarian Nordic of Denmark announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services (HHS), signed a contract with it to supply a freeze-dried version of its smallpox and monkeypox vaccine.4 A freeze-dried version of the vaccine is stated to have a longer shelf life.5
BARDA placed an $119 million order for 13 million freeze-dried doses of Bavarian Nordic’s smallpox and monkeypox vaccine known as JYNNEOS®. The contract states that the U.S. has an option to purchase additional vaccines worth $180 million if needed.6
What is Monkeypox?
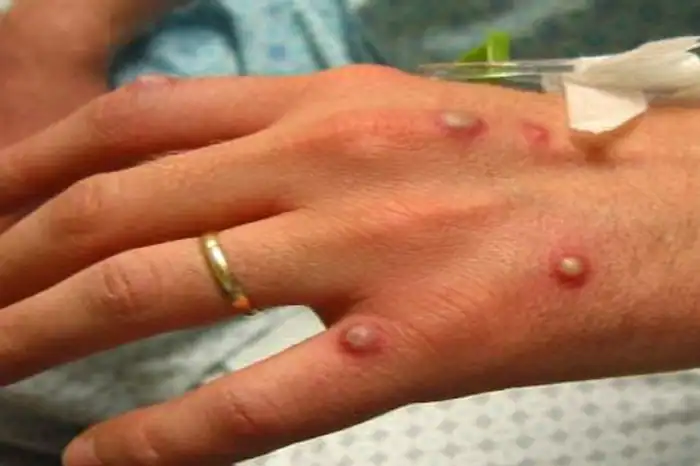
Monkeypox is a rare viral, zoonotic infectious disease that typically manifests with a fever, skin rashes, swollen lymph nodes, muscle and body aches, back pain, and extreme fatigue lasting approximately two to four weeks. Monkeypox is caused by the monkeypox virus, which is a member of the Orthopoxvirus genus in the Poxviridae family of viruses.7
Transmission of the monkeypox virus occurs through close contact with an infected person or animal or with material contaminated with the virus. Person to person transmission occurs through close contact with open skin lesions, body fluids, respiratory droplets and contaminated materials, such as bedding. According to the WHO, monkeypox is not as contagious as smallpox and usually causes less severe illness.8
FDA Approved JYNNEOS® Smallpox and Monkeypox Vaccine in 2019
On Sept. 24, 2019, the U.S. Food and Drug Administration (FDA) approved a live, non-replicating JYNNEOS® smallpox and monkeypox vaccine in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection. JYNNEOS® is a two-dose vaccine given four weeks apart. It is the only FDA approved vaccine in the U.S for the prevention of monkeypox disease.9
According to the FDA:
Jynneos does not contain the viruses that cause smallpox or monkeypox. It is made from a vaccinia virus, a virus that is closely related to, but less harmful than, variola or monkeypox viruses and can protect against both of these diseases. Jynneos contains a modified form of the vaccinia virus called Modified Vaccinia Ankara, which does not cause disease in humans and is non-replicating, meaning it cannot reproduce in human cells.10
First Case of Monkeypox Reported in the U.S.
On May 18, 2022, the Massachusetts Department of Public Health (MDPH) reported that a resident in the state has tested positive for monkeypox. The patient, who was admitted to Massachusetts General Hospital in Boston, is an adult male to recently returned from traveling to Canada.11
A MDPH press release stated:
DPH is working closely with the CDC, relevant local boards of health, and the patient’s health care providers to identify individuals who may have been in contact with the patient while he was infectious. This contact tracing approach is the most appropriate given the nature and transmission of the virus. The case poses no risk to the public, and the individual is hospitalized and in good condition.12
By May 7, there had been cases of monkeypox reported in Europe. An individual who had travelled to Nigeria, where monkeypox is endemic, reportedly was thought to have been the source of transmission.13
HHS Says Monkeypox Vaccine Purchase Not Related to the Outbreak
A spokesperson from HHS said that BARDA’s recent purchase of the smallpox and monkeypox vaccine JYNNEOS® is not related to the recent monkeypox outbreak. “The most recent BARDA purchase of smallpox vaccine was part of a standard and ongoing preparedness efforts and unrelated to specific events. BARDA has worked with industry to develop and purchase vaccines and treatments for a potential smallpox emergency, some of which may also be used to respond to monkeypox,” said an HHS spokesperson.14
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:
No comments:
Post a Comment