FDA Used ‘Critically Flawed’ Risk-Benefit Analysis to ‘Justify’ COVID Vaccines for Children
The U.S. Food and Drug Administration relied on a critically flawed risk-benefit assessment to authorize emergency use of Pfizer’s COVID-19 vaccine for children 5-11 years of age.
Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The U.S. Food and Drug Administration (FDA) on Oct. 29, 2021, granted Emergency Use Authorization (EUA) of Pfizer’s COVID-19 vaccine for children 5 to 11 years of age.
The Centers for Disease Control and Prevention (CDC) went on to recommend Pfizer’s COVID-19 vaccine for 28 million American children 5 to 11 years of age on Nov. 2, 2021.
This week, Pfizer asked the FDA to authorize the use of a two-dose vaccine in children 6 months to 4 years old. Data on a third shot would be submitted to regulators once they became available in the spring — clearing the way for the agency to authorize a three-shot regimen for the youngest children who can get vaccinated. The vaccine for this age group could be available as early as February.
The FDA and CDC safety surveillance systems have found an increased risk of heart inflammation (myocarditis/pericarditis) following vaccination with Pfizer’s COVID-19 vaccine. This observed risk was found to be highest in 12- to 17-year-old males, particularly following the second dose.
Vaccine risks versus benefits
For EUA to be issued for a vaccine, the FDA must determine the benefits of the vaccine outweigh the risks.
According to an FDA news release:
“FDA conducted its own benefit-risk assessment using modeling to predict how many symptomatic COVID-19 cases, hospitalizations, intensive care unit (ICU) admissions and deaths from COVID-19 the vaccine in children 5 through 11 years of age would prevent versus the number of potential myocarditis cases, hospitalizations, ICU admissions and deaths that the vaccine might cause. The FDA’s model predicts that overall, the benefits of the vaccine would outweigh its risks in children 5 through 11 years of age.”
Unfortunately, the FDA’s risk-benefit assessment was deeply flawed and failed to account for the following critically important factors:
1. Natural immunity
According to CDC data, an estimated 42% of children ages 5-11 had seroprevalence of previous COVID-19 infection as of June 2021. More recent estimates should suggest even higher seroprevalence rates among children, given that several months had passed since the time of this data point.
The FDA’s risk-benefit assessment failed to account for the large proportion of children in the United States who already had COVID-19, recovered from it and now have natural immunity.
For these millions of children, the risks of COVID-19 vaccination outweigh the benefits, as studies show natural immunity is superior to vaccine-induced immunity.
An FDA senior advisor for risk-benefit assessment admitted that should natural immunity be equal to vaccine-induced immunity, then that would result in a 45% reduction of all the benefits in the FDA’s risk-benefit analyses.
Males 5-11 years old (cases per million)

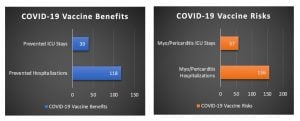
Using the FDA’s risk-benefit analysis (shown above) and conservatively adjusting for 42% of children having already acquired natural immunity through prior COVID-19 infection, the risk of hospitalization from vaccine-related heart inflammation in 5 to 11 year-old boys is greater than the number of COVID-19 hospitalizations prevented by vaccination.
As illustrated below, by adjusting for natural immunity (with a 42% reduction of vaccine benefits), 39 ICU stays are prevented by vaccination, but at the risk of 57 vaccine-related myopericarditis ICU stays.
Additionally, while 118 hospitalizations are prevented by vaccination, this is at the risk of 156 vaccine-related myopericarditis hospitalizations, for 5-11-year-old boys.
Males 5-11 years old (cases per million)
2. Underestimated myocarditis rates
The FDA’s risk-benefit analysis assumed an incidence rate of 106 myopericarditis cases per million children vaccinated. However, a Kaiser Permanente study found the actual myopericarditis incidence rate to be 208 cases per million children vaccinated.
The study authors write the following:
“The true incidence of myopericarditis is markedly higher than the incidence reported to US advisory committees… we identified that the encounter text description methodology identified approximately twice as many cases of myopericarditis following COVID-19 mRNA vaccination.”
Males and Females 5-11 years old (cases per million)
-

FDA’s “base” modeling scenario #1 risk-benefit analysis (males and females combined)
Using the FDA’s risk-benefit analysis (shown above) and correcting for the underestimated myopericarditis rate following vaccination (by using a multiplication factor of 1.96), we arrive at 67 ICU stays and 180 hospitalizations from myopericarditis per million children vaccinated.
By also adjusting for natural immunity (with a 42% reduction of vaccine benefits), we calculate that 36 ICU stays and 111 hospitalizations are prevented by vaccination. After correcting for the FDA’s underestimated myopericarditis rate and adjusting for natural immunity, we find that the risk of hospitalization from vaccine-related myopericarditis is greater than the number of COVID-19 hospitalizations prevented by vaccination, for boys and girls (as illustrated below).
Males and Females 5-11 years old (cases per million)
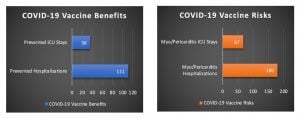
[Calculations: 62 prevented ICU stays x (1 – 0.42) natural immunity adjustment = 36 prevented ICU stays after adjustment. 192 prevented hospitalizations x (1 – 0.42) natural immunity adjustment = 111 prevented hospitalizations after adjustment. Multiplication factor of 1.96 = 208 (true incidence of myopericarditis per million children vaccinated) / 106 (FDA’s underestimated incidence of myopericarditis per million children vaccinated). 34 excess myopericarditis ICU stays x 1.96 (multiplication factor) = 67 vaccine-related myopericarditis ICU stays after adjustment. 92 excess myopericarditis hospitalizations x 1.96 (multiplication factor) = 180 vaccine-related myopericarditis hospitalizations after adjustment]
3. Over-classification of COVID-19 hospitalizations
Pediatric hospitalization rates, while used as a marker of COVID-19 disease severity in children, can be inflated by the detection of mild or asymptomatic infection via universal screening.
A Stanford University study found that 45% of pediatric COVID-19 hospital admissions were unlikely to have been caused by SARS-CoV-2.
According to a CDC medical officer, in COVID-NET data approximately 19% of younger children who were classified as COVID-19 hospital admissions were not primarily hospitalized due to COVID-19.
Inexplicably, the FDA did not adjust for this in their risk-benefit assessment.
4. Inflated COVID-19 hospitalization rates
According to COVID-NET data, as of December 25, 2021, the weekly rate of COVID-19 associated hospitalization ranged from zero to a peak of 1.1 per 100,000 children ages 5-11.
At the time of the FDA’s risk-benefit assessment, the overall weekly average COVID-19 associated hospitalization rate in this age group was approximately 0.4 per 100,000 children.
Rather than use the weekly average COVID-19 hospitalization rate since the start of the pandemic, the FDA’s risk-benefit assessment used an arbitrary average of the four weeks prior to September 11, 2021, resulting in a COVID-19 hospitalization rate of approximately 0.74 per 100,000 children (which is nearly double the average COVID-19 hospitalization rate of 0.4 per 100,000 children).
This further skewed the FDA’s risk-benefit analysis in favor of vaccination.
5. Waning immunity
The FDA’s risk-benefit assessment assumed a constant vaccine efficacy over a time span of six months. This was an inappropriate assumption, as it is well established that the effectiveness of Pfizer’s COVID-19 vaccine rapidly declines over time, with one study showing a drop below 50% effectiveness after five months.
Moreover, the FDA’s risk-benefit assessment did not account for a potentially necessary booster dose after five months. Each booster dose would carry an additional risk of myocarditis, along with the risk of other vaccine adverse events.
6. Vaccine adverse reactions
The FDA only accounted for risks from myocarditis/pericarditis following vaccination, but failed to account for adverse reactions and other adverse events in their risk-benefit assessment.
Over a six-month period, the FDA’s risk-benefit analysis estimated that 45,773 cases of COVID-19 could be prevented by fully vaccinating one million children ages 5-11.
However, this benefit of vaccination would be at the risk of an enormous number of vaccine adverse reactions (see section 7.6.2 Solicited adverse reactions): approximately 258,000 instances of moderate to severe injection site pain; 174,000 cases of fatigue; 94,000 headaches; 65,000 fevers; 55,000 cases of chills; 24,000 cases of diarrhea; 18,000 cases of vomiting; and 82,000 cases of muscle or joint pain.
These adverse reactions would be in addition to other adverse events, such as: anaphylaxis; lymphadenopathy; syncope; and Bell’s palsy.
Furthermore, data out of the UK has shown that individuals with prior COVID-19 infection are more likely to experience systemic side-effects following COVID-19 vaccination.
The opportunity cost
The FDA’s risk-benefit analysis estimated that fully vaccinating 1 million children ages 5-11 would prevent one COVID-19 death.
At a pandemic price of $19.50 per dose of Pfizer’s vaccine, it would cost $39 million to prevent a single COVID-19 death for a child in this age group.
This begs the question: How many more lives could be saved if this same sum of money were instead spent elsewhere?
Conclusion
The FDA relied upon a critically flawed risk-benefit assessment to justify EUA of Pfizer’s COVID-19 vaccine for children 5-11 years of age.
In the context of the Omicron variant, which is associated with less severe disease and lower hospitalization rates, the benefit-risk ratio of COVID-19 vaccination for children 5-11 years of age becomes even harder to justify.
Given all of the above, it is easy to understand why Sweden decided against recommending COVID-19 vaccines for children 5-11 years old. Arguing that the benefits did not outweigh the risks, a Swedish Health Agency official stated:
“With the knowledge we have today, with a low risk for serious disease for kids, we don’t see any clear benefit with vaccinating them.”
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views of Children's Health Defense.

