April 30, 2020
Coronavirus and Infection Control: What’s Going on with Testing?
By Mary Holland, Vice Chair and General Counsel, Children’s Health DefensePeople locked down in their homes for weeks and months are eager to resume their lives. Many public health agencies, including the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), state that such reopening requires extensive testing for coronavirus. It’s a business truism that to manage something, you must measure it. So the focus on testing makes sense.
Dr. Sin Hang Lee is a pathologist and research scientist based in Connecticut who has been producing molecular tests for diseases and conditions for many years, including Lyme disease and human
papilloma virus (HPV) infection. Dr. Lee trained and taught in some of the world’s most prestigious institutions and has published scores of scientific articles in peer-reviewed journals.
Dr. Lee is a hero to those concerned about HPV vaccine safety because he proved that the HPV vaccine contained HPV DNA fragments, something that Merck and the Food and Drug Administration (FDA) denied. These fragments were part of the reasons for so many adverse events. His research made the FDA come clean about the presence of the DNA in the vaccine, but the agency continued to deny its significance and harmful potential.
The need for accurate coronavirus testing is imperative. It is especially critical in nursing homes and institutions caring for elderly patients, so that false-positive patients are not housed with true-positive patients. It’s also essential to ensure that staff in direct contact with highly susceptible patients be infection-free.
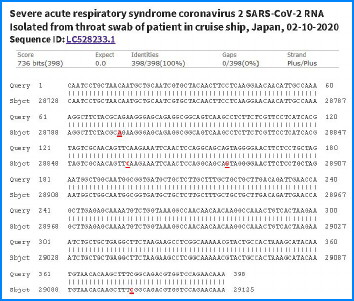
Dr. Lee has been working diligently to overcome the roadblocks in coronavirus testing – only to hit new ones at the very agencies responsible for ensuring the pandemic’s end. Dr. Lee wrote to the WHO
 and
to Dr. Anthony Fauci at the National Institute of Allergies and
Infectious Diseases of the National Institutes of Health (NIH) to
explain why the current tests to detect SARS-CoV-2 RNA are generating
false positives and negatives. He explained that a two-phased test would
“guarantee no-false positive results” based on his research and
published work from Japan.
and
to Dr. Anthony Fauci at the National Institute of Allergies and
Infectious Diseases of the National Institutes of Health (NIH) to
explain why the current tests to detect SARS-CoV-2 RNA are generating
false positives and negatives. He explained that a two-phased test would
“guarantee no-false positive results” based on his research and
published work from Japan.He offered to retest the residues of any patient samples that have generated questionable results, using a highly sensitive nested reverse transcription polymerase chain reaction (PCR) test followed by DNA sequencing of a unique 398 bp N gene amplicon for confirmation. Dr. Lee asked to be in contact with the coronavirus teams at the WHO and NIH so that he could assist them to ensure highly accurate tests, especially for elder care facilities where the need is greatest for extreme accuracy.
Given the specificity of his letter, with its charts on DNA sequencing, and its significance for preventing disease spread, it is almost unbelievable that as of April 27, Dr. Lee has received no response from either the WHO or NIH. He has similarly reached out to the Connecticut Department of Public Health to receive patient samples for further validation testing. The Connecticut Department has stopped responding to Dr. Lee’s emails and phone calls.
Please read Dr. Lee’s March 22 letter and decide for yourself if these agencies are serving the public interest or if perhaps they are serving competing or corrupt interests instead.
No comments:
Post a Comment