Of mice and men: on the origin of XMRV
- Laboratory of Experimental Virology, Department of Medical Microbiology, Center for Infection and Immunity Amsterdam, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
most controversial virus of this moment. After its original discovery in prostate cancer tissue from North American patients, it was subsequently detected in individuals with chronic fatigue syndrome from the same continent. However, most other research groups, mainly from Europe, reported negative results. The positive results could possibly be attributed to contamination with mouse products in a number of cases, as XMRV is nearly identical in nucleotide sequence to endogenous retroviruses in the mouse genome. But the detection of integrated XMRV proviruses in prostate cancer tissue proves it to be a genuine virus that replicates in human cells, leaving the question: how did XMRV enter the human population? We will discuss two possible routes: either via direct virus transmission from mouse to human, as repeatedly seen for, e.g., Hantaviruses, or via the use of mouse-related products by humans, including vaccines. We hypothesize that mouse cells or human cell lines used for vaccine production could have been contaminated with a replicating variant of the XMRV precursors encoded by the mouse genome.
Introduction
The xenotropic murine leukemia virus-related virus (XMRV)
is undoubtedly the most controversial human virus since its first
detection in human samples in 2006 (Urisman et al., 2006).
XMRV infection still lacks a firm disease association, although the
virus was originally isolated from prostate cancer tissue and
subsequently detected in the blood of American patients with chronic
fatigue syndrome (CFS; Lombardi et al., 2009), and in the respiratory tract of patients with or without a respiratory tract infection (Fischer et al., 2010). However, irregular XMRV detection (Fischer et al., 2008, 2010; Lombardi et al., 2009; Groom et al., 2010; Switzer et al., 2010; van Kuppeveld et al., 2010)
suggests that it is not likely to be a major causal factor. First, we
do not know whether the biological reservoir has been investigated thus
far, as most studies focused exclusively on blood or prostate tissue
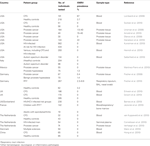
(summarized in Table 1).
Second, some pathogenic retroviruses do not cause much of a viremia,
and experimental infection of macaques suggests that this is also the
case for XMRV (Sharma et al., 2010).
In these monkeys, virus inoculation resulted in a low transient plasma
viremia, followed by a wide dissemination of replicating virus into
various organs including spleen, lymph nodes, and gastrointestinal
tract. Third, sequence variation may exist, but such variant virus
strains could be missed by the PCR primers used.
TABLE 1
Whether or not the virus causes disease in humans (reviewed extensively by Silverman et al., 2010, see also comments by Coffin and Stoye, 2009, by Kearney and Maldarelli, 2010, by Kaiser, 2010, and the cautionary note by Weiss, 2010), and how and when XMRV entered the human population – as the first Gammaretrovirus
to do so – remains unclear. To add to the ongoing discussion, we would
like to propose an alternative possible source for XMRV, human vaccines
or other biological products that were produced in murine cells.
How did XMRV Enter the Human Population?
One of the most striking aspects of XMRV biology is the
high sequence similarity to mouse chromosomal sequences that encode
endogenous retroviruses. Initially, this raised the speculation that
contamination with mouse DNA could explain the presence of XMRV in human
samples. However, the absence of other mouse-derived sequences,
combined with the ease of infection of human cells with XMRV in vitro (Stieler et al., 2010), and the detection of integrated proviruses in prostate cancer tissues (Dong et al., 2007; Kim et al., 2008)
indicated that laboratory contamination with mouse products is not a
likely explanation for the origin of XMRV, at least for some of these
studies. If contamination does not provide an explanation, where does
the virus come from and how did it end up in humans?
Direct transmission of viruses from wild rodents to humans is not uncommon, e.g., rodent Hantaviruses and Arenaviruses spread through excrement via aerosols and are able to infect non-rodent species, including humans (Hart and Bennett, 1999; Klein and Calisher, 2007; Charrel and de Lamballerie, 2010).
Transmission of xenotropic murine leukemia viruses (X-MLV’s) to humans
is possible as human cells do express the XPR1 protein that is able to
function as receptor for xenotropic and polytropic murine retroviruses.
The human XPR1 receptor protein shows a preference for xenotropic
retroviruses, but is also able to mediate infection of polytropic murine
leukemia retroviruses (P-MLV’s; Tailor et al., 1999). Classical laboratory mice strains are hybrids between Mus musculus musculus, M. m. domesticus and M. m. castaneus, with around two-thirds of the genome coming from M. m. domesticus (Yang et al., 2007).
X-MLV’s cannot (re-)infect most of the laboratory mouse strains due to
polymorphisms in the XPR1 protein that disable xenotropic virus entry (Marin et al., 1999). Interestingly, the XPR1 genotype that prohibits X-MLV entry was not found in wild-caught M. m. domesticus, suggesting that it is a rare allele (Baliji et al., 2010).
Indeed, extensive screening identified seven strains of laboratory mice
strains containing a permissive allele, of which at least three were
susceptible to X-MLV and XMRV in cell culture (Baliji et al., 2010). In addition, the F/St mouse strain also produced infectious X-MLV together with a life-long viremia (Baliji et al., 2010). Many feral mice species, e.g., M. dunni and M. spretus, are also susceptible to infection with X-MLV’s (Battini et al., 1999; Marin et al., 1999; Tailor et al., 1999). Evidence on M. m. castaneus is conflicting, with some reporting a non-functional and others a susceptible XPR1 phenotype (Marin et al., 1999; Yan et al., 2010).
The ability of XPR1 to function as a receptor for xenotropic viruses
was found to depend on the identity of two amino acid residues (Marin et al., 1999).
The Origin of XMRV?
Every mouse genome contains multiple copies of
endogenous MLV and has thus the capacity to express viral RNA and
possibly viral particles. Endogenous MLV transcription has been
described for many tissues and several mouse strains. It remains unclear
if and when virus particles are generated and whether these particles
are actually excreted. Zoonotic transmission of these viruses could have
occurred in the many million years that mice and men have shared the
same environment. But current XMRV sequences isolated from human samples
do closely mimic mouse genomic sequences, thus suggesting a low number
of replication cycles since zoonotic transmission, which is thus likely
to have occurred recently. The mutation rate of MLV’s is not different
from other retroviruses (Sanjuan et al., 2010;
although its replication rate may be low), implying that if the
transmission had taken place a long time ago, more nucleotide
substitutions should have become fixed. Phylogenetic sequence analysis,
however, revealed very short branches for XMRV and the mouse xMERV
sequences on chromosomes 7 and 9, indicating that very few mutations
have occurred since transmission (Urisman et al., 2006; Fischer et al., 2008, 2010). In addition to the loci on chromosomes 7 and 9, a BLAST search using the NCBI sequence database1
retrieves loci on mouse chromosomes 4, 11, and 12 with a much higher
(98–100%) sequence identity to XMRV-gag nucleotide sequences (e.g.,
GenBank accession numbers AC124739, AY349138, and AL627314). Blasting
whole genome XMRV sequences recovers very similar sequences with large
stretches of sequence identity on mouse chromosomes 4, 5, 13, and Y,
especially for the 3′ end of the XMRV genome. These results suggest that
the genome of human XMRV is present, albeit in two parts, in the mouse
genome with effectively no nucleotide changes. Even in slowly evolving
retroviruses like foamy viruses, 100% sequence identity is only seen in
animals with close contact or humans that have been bitten by an
infected primate, suggestive of direct transmission, while intraspecies
variation is generally around 85–95% for the pol gene (Switzer et al., 2004; Calattini et al., 2006; Liu et al., 2008).
A recently described locus (Baliji et al., 2010) on chromosome 1 of M. musculus
(GenBank accession number AC115959) contains a provirus that is 92%
homologous to XMRV from the 22Rv1 cell line (GenBank accession number
FN692043) over its complete genome length. This provirus, Bxv1, is
mainly found in Japanese M. molossinus (a natural hybrid of M. castaneus and M. musculus) and is highly expressed in some laboratory mouse strains (Baliji et al., 2010).
However, the Bxv1 provirus is less likely to be the source of XMRV, as
its similarity to XMRV is much lower than that of other murine loci.
XMRV is a Novel Recombinant Retrovirus
Xenotropic murine leukemia virus-related virus is
actually a recombinant virus, resembling polytropic-endogenous sequences
for the 5′ half up to approximately the middle of the pol gene and xenotropic-endogenous sequences for the 3′ half of the genome, which includes the env gene (see: Courgnaud et al., 2010).
This recombination event is likely to have occurred in the mouse before
transmission to humans. At least one recombinant provirus, Bxv1, is
already found in the M. musculus genome (Baliji et al., 2010). This locus is heterogeneous in subspecies of M. musculus,
suggesting that it represents a recent integration. Recombination rates
are high for all retroviruses because they package two copies of the
RNA genome in virions, which drives subsequent mixing of sequences
during the reverse transcription process. Recombination also enables the
generation of replication-competent viruses from defective endogenous
proviruses. Recombination can also extend the viral host range (cell
type and/or host species). A virus carrying a xenotropic env gene
is more infectious for human cells as the human XPR1 protein has a
preference for xenotropic murine envelope proteins over polytropic ones.
The replication-competent endogenous cat retrovirus RD-114 is an
example of a recombinant virus expressed from endogenous sequences. It
combines FcEV gag–pol genes (FcEV is an endogenous retrovirus of cats) and a BaEV env gene (BaEV is an endogenous retrovirus of African monkeys) (van der Kuyl et al., 1999). RD-114 is expressed by all species of the genus Felis,
but not in other felines, and probably originates from a cross-species
transmission of BaEV, followed by a recombination event and subsequent
germ-line integration.
Are Mouse-Derived Biological Products the Source of XMRV?
Detection rates of XMRV in populations are extremely
variable, with 0–67% positivity in patients and 0–3.7% in healthy
controls (Fischer et al., 2008, 2010; Lombardi et al., 2009; Groom et al., 2010; Switzer et al., 2010; van Kuppeveld et al., 2010),
suggesting that virus prevalence and thus exposure could vary
significantly with geographic location. Although the virus could
possibly be transmitted from feral mice to humans in a natural setting,
followed by a rapid dissemination in the human population, the high XMRV
sequence similarity on two continents would suggest an alternative
transmission route. Likely sources of XMRV are mouse-derived products.
Some mouse genomes encode complete copies of X-MLV’s with at least 92%
similarity to XMRV; segments with even higher homology are present on
other locations, and could result in novel recombinant viruses. So,
X-MLV’s that closely resemble XMRV could then be produced from these
loci and virions could be excreted from mouse tissue or cell cultures.
Are X-MLV’s Produced in Mice?
MLV’s, including xenotropic sequences, are actively transcribed in mouse brain (Kwon et al., 2008), and mice can produce virus particles of different MLV classes (Ribet et al., 2008). In vivo
recombination between endogenous and exogenous polytropic MLV’s has
also been reported, resulting in viable viral offspring capable of
infecting a variety of species (Evans et al., 2009). The Bxv1 locus in M. musculus molossinus
is an example of an endogenous xenotropic/polytropic recombinant MLV
that is expressed and gives rise to a life-long viremia in laboratory
mice of the F/St strain (Baliji et al., 2010).
Although there was no evidence of X-MLV transmission to
human embryonic stem cells expressing XPR1 after cocultivation with
murine cells expressing X-MLV particles in a single report (Amit et al., 2005),
this does not imply that transmission may not have occurred on another
occasion. The prostate carcinoma cell line 22Rv1 is a popular research
tool because it contains approximately 10 integrated copies of the XMRV
provirus and it produces infectious virus (Knouf et al., 2009).
The origin of the 22Rv1 cell line may represent a recent transmission
case as a carcinoma was grafted in nude mice to establish this permanent
cell line (Sramkoski et al., 1999). The complete 22Rv1 provirus has 99% sequence similarity with other XMRV isolates (Paprotka et al., 2010). Possibly, the 22Rv1 carcinoma cells were infected with XMRV by mouse cells surrounding the tumor graft (Knouf et al., 2009).
Vaccines, Viruses, and Contamination
One of the most widely distributed biological products
that frequently involved mice or mouse tissue, at least up to recent
years, are vaccines, especially vaccines against viruses. Some, for
instance vaccines against rabies virus (Plotkin and Wiktor, 1978), yellow fever (YF) virus (Frierson, 2010), and Japanese encephalitis (JE) virus (Inactivated Japanese Encephalitis Virus Vaccine, 1993),
consisted of viruses that were cultured on mouse brains. Such vaccines
were in use from 1931 (YF vaccine) until now (JE vaccine, licensed in
Japan since 1954). For rabies virus, early vaccines were mainly of goat
or sheep nerve tissue origin. In addition, suckling mouse brain-derived
rabies virus vaccines were used in South America and France (Plotkin and Wiktor, 1978). No mouse-derived rabies vaccine was ever licensed in the USA (Dennehy, 2001).
Live-attenuated YF vaccines were originally also grown on mouse brain,
but an YF vaccine grown on chicken eggs (named 17D) became available in
1937, and was since the vaccine of choice in the America’s. In 1962,
contamination of the 17D vaccine with oncogenic avian leukosis virus was
detected both in England and in the USA, but fortunately no excess of
cancer incidence among vaccines was reported (Frierson, 2010). In France, the mouse brain-derived YF vaccine was discontinued as late as 1982.
Although being the most effective means to prevent
infectious diseases and to safe lives, serious contamination problems
involving vaccines have occurred (Pastoret, 2010).
Contamination with unrelated viruses such as the presence of hepatitis B
virus (HBV) in YF vaccine preparations stemming from the use of human
serum for stabilization, and simian virus 40 (SV40) and foamy viruses
through the use of monkey cell cultures (Pastoret, 2010).
Some vaccine viruses are inactivated before use, hopefully also
inactivating any contaminating virus particles, but the contaminating
virus may be more stable than the vaccine virus. For instance, SV40 is
highly resistant to inactivation (Murray, 1964).
Endogenous retroviruses constitute a distinct class of contaminating
viruses, as these viruses are encoded by all cells of a certain species,
and therefore cannot be avoided even through rigorous screening (Miyazawa, 2010).
Contamination with endogenous avian leukosis viruses is a major problem
for vaccine viruses grown in chicken embryos or chicken embryonic
fibroblasts (Hussain et al., 2003). Infectious cat endogenous RD-114 virus has been found in several veterinary vaccines produced in cat cell cultures (Miyazawa et al., 2010; Yoshikawa et al., 2010).
XMRV and Monoclonal Antibodies
Apart from vaccines, other mouse-derived biologicals
could have been a source of XMRV in the human population. Monoclonal
antibodies present a modern treatment for many cancers and other
diseases including cardiovascular disease, psoriasis, and auto-immune
disorders (for a review see: Stern and Herrmann, 2005).
The first monoclonal antibody, OKT3 (to be used against transplant
rejection), was approved by the FDA in 1986. The market for monoclonal
antibody therapy has been expanding rapidly after the year 2000.
Initially, murine antibodies produced by the hybridoma technique were
used (Kohler and Milstein, 1975),
but these have been largely abandoned because of (sometimes severe)
allergic reactions. The murine antibodies were often replaced by
humanized antibodies mainly produced in transgenic mice. Monoclonal
antibodies generated in mice could possibly be polluted by XMRV and
related viruses. Platinum Taq polymerase from Invitrogen Corporation,
prepared using mouse monoclonal antibodies, is known to be frequently
contaminated with mouse DNA, which can generate false-positive PCR
amplifications in combination with X-MLV or XMRV primers (Erlwein et al., 2010a).
It is less likely that monoclonal antibodies from mice are a major
source of XMRV in the human population as they are in use only recently,
but they could provide a future supply of mouse-derived viruses.
Although monoclonals are treated with detergents before use in patients,
virus inactivation may not be complete, especially as protein function
should be conserved. And if retroviral particles containing RNA genomes
are copurified with the antibody proteins, the absence of mouse DNA may
give a false impression of safety.
XMRV Contamination of Cell Lines?
It is possible that XMRV particles were present in virus
stocks cultured in mice or mouse cells for vaccine production, and that
the virus was transferred to the human population by vaccination. The
sequence homogeneity of all XMRV isolates known today suggests that only
a single or very few transmissions have occurred, which is consistent
with the proposed vaccination route. Nowadays, vaccine batches are
carefully checked with sensitive PCR assays for the presence of
contaminating retroviruses, but this screening was not performed in the
early years of vaccination (Trijzelaar, 1993 see also Miyazawa et al., 2010).
Apart from vaccines, other biological products have been generated
using mice or mouse cells. Alternatively, laboratory contamination with a
mouse-derived virus of cell lines used for, e.g., vaccine production
could have occurred (Hartley et al., 2008; Takeuchi et al., 2008; Stang et al., 2009).
The virus could then unintentionally have been transmitted to the human
population. Nowadays, many vaccine strains are grown in human diploid
cell lines (Fletcher et al., 1998),
which are susceptible to MLV infection. A recent report detected other
MLV-related sequences in CFS patients and healthy controls from North
America (Lo et al., 2010),
suggesting that more MLV strains may have been transmitted to the human
population, possibly in a similar fashion. However, solid evidence that
these polytropic MLV sequences represent replicating virus is currently
lacking.
Where was XMRV Transmitted?
Xenotropic murine leukemia virus-related virus was found
in samples from CFS patients in North America, but not in Europe. The
virus was detected in prostate cancer tissue from patients on both
continents. There is a single report with negative results from China (Hong et al., 2010), and a single report with one positive sample from Mexico (Martinez-Fierro et al., 2010)
but none from other areas of the world, leaving many questions about
the true distribution of XMRV in humans. Prevalence of XMRV from North
American studies varies between 3.7 and 67% in four studies with two
other studies reporting negative results (one in CFS patients and
healthy controls (Switzer et al., 2010), and one in HIV-infected patients receiving antiretroviral therapy and untreated men at risk for HIV infection (Kunstman et al., 2010)). In Europe, XMRV was detected in two studies from Germany (Fischer et al., 2010), and in one from The Netherlands (Verhaegh et al., 2010), but not in the UK (Erlwein et al., 2010b; Groom et al., 2010), France (Jeziorski et al., 2010), Denmark (Maric et al., 2010), and two other studies from The Netherlands (Cornelissen et al., 2010; van Kuppeveld et al., 2010), although the nature of the samples analyzed differed between studies. Table 1 summarizes the results from these studies.
Xenotropic murine leukemia virus-related virus sequences
from Germany and North America exhibit very little nucleotide
divergence, suggesting that they descended from a common ancestor
relatively recently. A close inspection of the phylogenetic trees
obtained with XMRV-gag sequences (Fischer et al., 2008, 2010)
suggests that XMRV sequences from the USA are closer to the common
ancestor than German XMRV sequences, although the trees are not optimal
due to the high sequence conservation. Being closer to the most recent
common ancestor (MRCA) is suggestive of an older virus. Possibly, XMRV
was transmitted from mice to men in the USA, and soon after this event
introduced into Germany. Germany had close connections with the USA
after World War II, with large numbers of military personnel (and their
families) stationed in Germany from 1945 till present times. In 2006,
there were still 57.080 American army employees distributed over more
than 200 locations in Germany, mainly in the south and west of the
country2.
US military personnel are highly vaccinated, e.g., virtually all
recruits were vaccinated with YF vaccine in 1941–1942 after the outbreak
of World War II (Frierson, 2010).
A massive outbreak of jaundice, with at least 26,000 cases in the
Western region of the USA, was due to the use of human serum
contaminated with HBV in the vaccine (see Frierson, 2010). Recently, massive smallpox vaccination of the US army personnel has been carried out (Grabenstein and Winkenwerder, 2003). XMRV-infected Americans could subsequently have introduced the virus into Germany.
Spread of XMRV
The combined results suggest (1) that XMRV was recently
transmitted from mice to humans, either from a single source, or at
least from a single (sub) species of mice, and (2) that all
XMRV-positive individuals known today were infected with this newly
emerged virus only recently, as a very high sequence identity is
normally only seen after a direct retrovirus transmission.
Whatever the mechanism of XMRV cross-species
transmission from mouse in humans, the possible spread from human to
human forms a major health threat. Sexual transmission was initially
proposed (Hong et al., 2009), but XMRV was not detected in seminal plasma from HIV-infected men (Cornelissen et al., 2010). The detection of XMRV fragments in the respiratory tract (Fischer et al., 2010)
suggests that the virus may be transmitted by saliva, although RNA
concentrations were low. Transmission through saliva, mainly by biting,
has been reported for most retrovirus genera, including ecotropic MLV’s (Portis et al., 1987). Another major threat is transmission through blood products as infectious virus has been cultured from blood cells (Lombardi et al., 2009).
Up till now, all patients with detectable XMRV have been
adults, the majority of them middle-aged or older (mean ± 55 years). A
study in 142 children with a diversity of pathologies, including
respiratory diseases in France revealed no XMRV infections in that age
group (Jeziorski et al., 2010),
although the incidence of XMRV in France is not known. Another study in
autistic children from the USA and Italy was also negative for XMRV (Satterfield et al., 2010).
XMRV can likely be acquired at any age, and then probably establishes a
chronic, latent infection like other retroviruses. Therefore the age of
XMRV-infected individuals does not provide an unambiguous clue about
when XMRV entered the human population.
Conclusion
In conclusion, the most likely mode of XMRV transmission
points to mouse-derived biological products, but it cannot formally be
excluded that the virus was once transferred from feral mice to humans.
The latter scenario is less likely as it would imply that a very rapid
spread in the human population must have occurred to explain its
presence on two continents. In this scenario, the extreme sequence
similarity among XMRV genomes, both between and within individuals,
would argue that the virus replicates at very low levels. Among the
biological products, vaccines that were produced in mice or mouse cells
are possible candidates that warrant further inspection. If XMRV was
introduced in the human population through the use of biologicals, a
background level of the virus in the human population, possibly varying
with geography or age group, would be expected. Such a low level
presence would then also explain the (absence of) detection of the virus
in different studies, as well as its controversial association with
disease.
We hope that this hypothesis will spur further discussion and help to resolve the many remaining XMRV questions.
Conflict of Interest Statement
The authors declare that the research was conducted in
the absence of any commercial or financial relationships that could be
construed as a potential conflict of interest.
Acknowledgment
We thank Hans Zaaijer for insightful discussions and proofreading of the manuscript.
Footnotes
References
Arnold, R. S.,
Makarova, N. V., Osunkoya, A. O., Suppiah, S., Scott, T. A., Johnson, N.
A., Bhosle, S. M., Liotta, D., Hunter, E., Marshall, F. F., Ly, H.,
Molinaro, R. J., Blackwell, J. L., and Petros, J. A. (2010). XMRV
infection in patients with prostate cancer: novel serologic assay and
correlation with PCR and FISH. Urology 75, 755–761.
Barnes, E.,
Flanagan, P., Brown, A., Robinson, N., Brown, H., McClure, M., Oxenius,
A., Collier, J., Weber, J., Günthard, H. F., Hirschel, B., Fidler, S.,
Phillips, R., and Frater, J. (2010). Failure to detect xenotropic murine
leukemia virus related virus in blood of individuals at high risk of
blood borne viral infections. J. Infect. Dis. 202, 1482–1485.
Danielson, B. P.,
Ayala, G. E., and Kimata, J. T. (2010). Detection of xenotropic murine
leukemia virus related virus in normal and tumor tissue of patients from
the Southern United States with prostate cancer is dependent on
specific polymerase chain reaction conditions. J. Infect. Dis. 202, 1470–1477.
Henrich, T. J.,
Li, J. Z., Felsenstein, D., Kotton, C. N., Plenge, R. M., Pereyra, F.,
Marty, F. M., Lin, N. H., Grazioso, P., Crochiere, D. M., Eggers, D.,
Kuritzkes, D. R., and Tsibris, A. M. N. (2010). Xenotropic murine
leukemia virus related virus prevalence in patients with chronic fatigue
syndrome or chronic immunomodulatory conditions. J. Infect. Dis. 202, 1478–1481.
Hong, S., Klein,
E. A., Das, G. J., Hanke, K., Weight, C. J., Nguyen, C., Gaughan, C.,
Kim, K. A., Bannert, N., Kirchhoff, F., Munch, J., and Silverman, R. H.
(2009). Fibrils of prostatic acid phosphatase fragments boost infections
with XMRV (xenotropic murine leukemia virus-related virus), a human
retrovirus associated with prostate cancer. J. Virol. 83, 6995–7003.
Kim, S., Kim, N.,
Dong, B., Boren, D., Lee, S. A., Das, G. J., Gaughan, C., Klein, E. A.,
Lee, C., Silverman, R. H., and Chow, S. A. (2008). Integration site
preference of xenotropic murine leukemia virus-related virus, a new
human retrovirus associated with prostate cancer. J. Virol. 82, 9964–9977.
Knouf, E. C.,
Metzger, M. J., Mitchell, P. S., Arroyo, J. D., Chevillet, J. R.,
Tewari, M., and Miller, A. D. (2009). Multiple integrated copies and
high-level production of the human retrovirus XMRV (xenotropic murine
leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J. Virol. 83, 7353–7356.
Liu, W., Worobey,
M., Li, Y., Keele, B. F., Bibollet-Ruche, F., Guo, Y., Goepfert, P. A.,
Santiago, M. L., Ndjango, J. B., Neel, C., Clifford, S. L., Sanz, C.,
Kamenya, S., Wilson, M. L., Pusey, A. E., Gross-Camp, N., Boesch, C.,
Smith, V., Zamma, K., Huffman, M. A., Mitani, J. C., Watts, D. P.,
Peeters, M., Shaw, G. M., Switzer, W. M., Sharp, P. M., and Hahn, B. H.
(2008). Molecular ecology and natural history of simian foamy virus
infection in wild-living chimpanzees. PLoS Pathog. 4, e1000097. doi: 10.1371/journal.ppat.1000097
Lombardi, V. C.,
Ruscetti, F. W., Das, G. J., Pfost, M. A., Hagen, K. S., Peterson, D.
L., Ruscetti, S. K., Bagni, R. K., Petrow-Sadowski, C., Gold, B., Dean,
M., Silverman, R. H., and Mikovits, J. A. (2009). Detection of an
infectious retrovirus, XMRV, in blood cells of patients with chronic
fatigue syndrome. Science 326, 585–589.
Martinez-Fierro,
M. L., Leach, R. J., Gomez-Guerra, L. S., Garza-Guajardo, R.,
Johnson-Pais, T., Beuten, J., Morales-Rodriguez, I. B.,
Hernandez-Ordonez, M. A., Calderon-Cardenas, G., Ortiz-Lopez, R.,
Rivas-Estilla, A. M., ncer-Rodriguez, J., and Rojas-Martinez, A. (2010).
Identification of viral infections in the prostate and evaluation of
their association with cancer. BMC Cancer 10, 326. doi: 10.1186/1471-2407-10-326
Sharma, P.,
Suppiah, S., Molinaro, R., Rogers, K., Das Gupta, J., Silverman, R.,
Hackett, J. Jr., Devare, S., Schochetman, G., and Villinger, F. (2010).
“Organ and cell lineage dissemination of XMRV in rhesus macaques during
acute and chronic infection [abstract],” in CROI, San Francisco, February 16–19, 2010, Paper #150LB.
Switzer, W. M.,
Jia, H., Hohn, O., Zheng, H., Tang, S., Shankar, A., Bannert, N.,
Simmons, G., Hendry, R. M., Falkenberg, V. R., Reeves, W. C., and
Heneine, W. (2010). Absence of evidence of xenotropic murine leukemia
Virus-related virus infection in persons with chronic fatigue syndrome
and healthy controls in the United States. Retrovirology 7, 57.
Urisman, A.,
Molinaro, R. J., Fischer, N., Plummer, S. J., Casey, G., Klein, E. A.,
Malathi, K., Magi-Galluzzi, C., Tubbs, R. R., Ganem, D., Silverman, R.
H., and DeRisi, J. L. (2006). Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2, e25. doi: 10.1371/journal.ppat.0020025
van Kuppeveld, F.
J., de Jong, A. S., Lanke, K. H., Verhaegh, G. W., Melchers, W. J.,
Swanink, C. M., Bleijenberg, G., Netea, M. G., Galama, J. M., and van
der Meer, J. W. (2010). Prevalence of xenotropic murine leukaemia
virus-related virus in patients with chronic fatigue syndrome in the
Netherlands: retrospective analysis of samples from an established
cohort. BMJ 340, c1018.
Citation: van der Kuyl AC, Cornelissen M and Berkhout B (2011) Of mice and men: on the origin of XMRV. Front. Microbio. 1:147. doi: 10.3389/fmicb.2010.00147
Received: 20 September 2010;
Accepted: 26 December 2010;
Published online: 17 January 2011.
Published online: 17 January 2011.
Edited by:
Antti Vaheri, University of Helsinki, Finland
Antti Vaheri, University of Helsinki, Finland
Reviewed by:
Jonas Blomberg, Uppsala University and Uppsala Academic Hospital, Sweden
Nicole Fischer, University Medical Center Hamburg-Eppendorf, Germany
Copyright: © 2011 van der Kuyl, Cornelissen and
Berkhout. This is an open-access article subject to an exclusive license
agreement between the authors and the Frontiers Research Foundation,
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original authors and source are credited.Jonas Blomberg, Uppsala University and Uppsala Academic Hospital, Sweden
Nicole Fischer, University Medical Center Hamburg-Eppendorf, Germany
*Correspondence: Ben Berkhout, Laboratory of Experimental Medicine, Department of Medical Microbiology, Academic Medical Center, Meibergdreef 15, 1105 AZ Amsterdam, Netherlands. e-mail: b.berkhout@amc.uva.nl
No comments:
Post a Comment