Share
Leave a comment
https://rumble.com/v4i968r-reject-the-amendments.html
 | Top 10 Issues That Are Unacceptable 125KB ∙ PDF file |
|
 | Updated Amendments To The International ... 4.34MB ∙ PDF file |
|
Regular text below is from the IHR(2005).
Bold text below is proposed additional amendments.
Struck through text below is text to be deleted.
"early
action alert" means an alert on an event which has not been declared a
public health emergency of international concern, at the time of
communication
to
potentially require coordinated international action to control spread
because the event has a potentially significant localized acute public
health impact in a limited number of countries and:
to constitute a potential risk of international spread and becoming a PHEIC.
“public
health emergency of international concern” means an extraordinary event
which is determined, as provided in these Regulations:
(i) to constitute a public health risk to other States through the international spread of disease and
(ii) to potentially require a coordinated international response;
"pandemic
emergency" means a public health emergency of international concern,
that is infectious in nature, in relation to which it is determined, in
accordance with Article 12, that the event:
(i) is significantly impacting or is likely to significantly impact various geographic regions around the globe; and:
(ii)
is causing or is likely to cause substantial disruptions to social,
economic, and health systems, including to travel and trade; and
(iii)
requires rapid and enhanced coordinated international action, with
global equity based and whole-of-society approaches because the event
has continued to spread despite established public health interventions.
1. Each State Party shall designate or establish one or
two entities, in accordance with its national law and context, to serve
as National IHR Authority and National IHR Focal Point, as well as the
authorities responsible within its respective jurisdiction for the
implementation of health measures under these Regulations.
1.
bis The National IHR Authority shall coordinate the implementation of
these Regulations within the territory of the State Party.
2.
bis States Parties shall take measures to implement paragraphs 1, 1.
bis, and 2 of this Article, including, as necessary, by allocating human
and financial resources and adjusting their national law, domestic
legislative and administrative arrangements in accordance with paragraph
3 of Article 59.
3.
State Parties with more resources shall make available additional
resources to WHO for assisting developing countries to develop,
strengthen and maintain the capacities.
5.
WHO should develop a early warning criteria for assessing and
progressively updating the national, regional, or global risk posed by
an event of known or unknown causes or sources and shall convey this
risk assessment to states parties in accordance with Article 11 & 45
where appropriate.
In
the case of events occurring within its territory not requiring
notification as provided in Article 6, in particular including those
events for which there is insufficient information available to complete
the decision instrument in order to assess the event within 48 hours in accordance with paragraph 6(a) of Annex 1, a State Party is strongly encouraged to nevertheless consider, whenever appropriate, keeping WHO advised thereof in a timely manner through the National IHR Focal Point and consult with WHO on appropriate health measures within 72 hours of the event being reported.
Such communications shall be treated in accordance with paragraphs 2 to
4 of Article 11. The State Party in whose territory the event has
occurred may request WHO assistance to assess any epidemiological
evidence obtained by that State Party.
1.
The Director-General shall determine, on the basis of the information
received, in particular from the States Parties within whose territory
an event is occurring, whether an event calls for an early action alert or constitutes a public health emergency of international concern, including, when appropriate, a pandemic emergency, in accordance with the criteria and the procedure set out in these Regulations.
New
5. The Director-General shall also determine whether a public health
emergency of international concern also constitutes a pandemic
emergency.
1.
Developed country State Parties and WHO undertake to provide resources
to developing countries for the building of capacities pursuant to this
provision equivalent to the amount of resources they provided to build
capacities under Article 5.
5. When requested by WHO, States Parties shall should provide, to the extent possible, support to WHO-coordinated response activities, including
supply of health products and technologies, especially diagnostics and
other devices, personal protective equipment, therapeutics, and
vaccines, for effective response to PHEIC occurring in another State
Party’s jurisdiction and/or territory, capacity building for the
incident management systems as well as for rapid response teams. Any
State Party unable to fulfill such requests shall inform the reasons for
the same to WHO and the Director General shall include the same in the
report submitted to WHA under Article 54 of these Regulations.
2bis.
When communicating the issuance, modification or extension of temporary
recommendations, the Director-General shall endeavor to adopt measures
and provide information to States Parties on access to, and availability
of health products, technologies and know-how through WHO-coordinated
mechanisms for fair and equitable access.
2.
When communicating the issuance, modification or extension of standing
recommendations, the Director-General shall endeavor to adopt measures
and provide information to States Parties on access to, and availability
of, health products, technologies and know-how through WHO-coordinated
mechanisms for fair and equitable access.
When issuing, modifying or terminating temporary or standing recommendations, the Director-General shall consider:
h)
the availability of relevant health products, technologies and
know-how, including in the context of WHO-coordinated access and
allocation mechanisms for a fair and equitable access.
1. States Parties shall take all practicable measures consistent with these Regulations to ensure that conveyance operators:
(a) comply with the health measures which may include isolation and quarantine recommended by WHO and adopted by the State Party including for
application on board as well as during embarkation and disembarkation;
Such measures, including isolation and quarantine, shall be based on
available evidence;
(b) inform travelers of the health measures recommended by WHO and adopted by the State Party including for application on board as well as during embarkation and disembarkation;
[From the International Health Regulations (2005)
“conveyance” means an aircraft, ship, train, road vehicle or other means of transport on an international voyage;
“conveyance operator” means a natural or legal person in charge of a conveyance or their agent;]
The competent authority may implement additional health measures, including isolation and quarantine of the conveyances, as necessary, to prevent the spread of disease.
2.
Health documents under these Regulations may be issued on non-digital,
digital or any other possible format, subject to the obligations of any
State Party regarding the format of such documents deriving from other
international agreements.
3.
Regardless of the format in which relevant health documents under these
Regulations have been issued, each State Party shall accept health
documents issued by other States Parties, as long as the health
documents conform to the Annexes referred to in Articles 36 to 39 and
their authenticity can be ascertained.
4.
WHO, in consultation with States Parties, shall develop and update, as
necessary, technical guidance, including specifications or standards
related to the issuance and ascertainment of authenticity of health
documents in digital format, as well as non-digital format. Such
specifications or standards shall be in accordance with Article 45
regarding treatment of personal data, and supporting the progressive
achievement of the interoperability of information technology platforms.
3.
In accordance with Article 35, other types of proofs and certificates,
such as test certificates, issued in conformity with Annex XXX, may be
considered by States Parties when accepting the entry of travelers into
their territories, in particular when a vaccine or prophylaxis has not
yet been made available for a disease in respect to which a public
health emergency of international concern has been declared.
1.
The master of a ship, before arrival at its first port of call in the
territory of a State Party, shall ascertain the state of health on
board, and, except when that State Party does not require it, the master
shall, on arrival, or in advance of the vessel’s arrival if the vessel
is so equipped and the State Party requires such advance delivery,
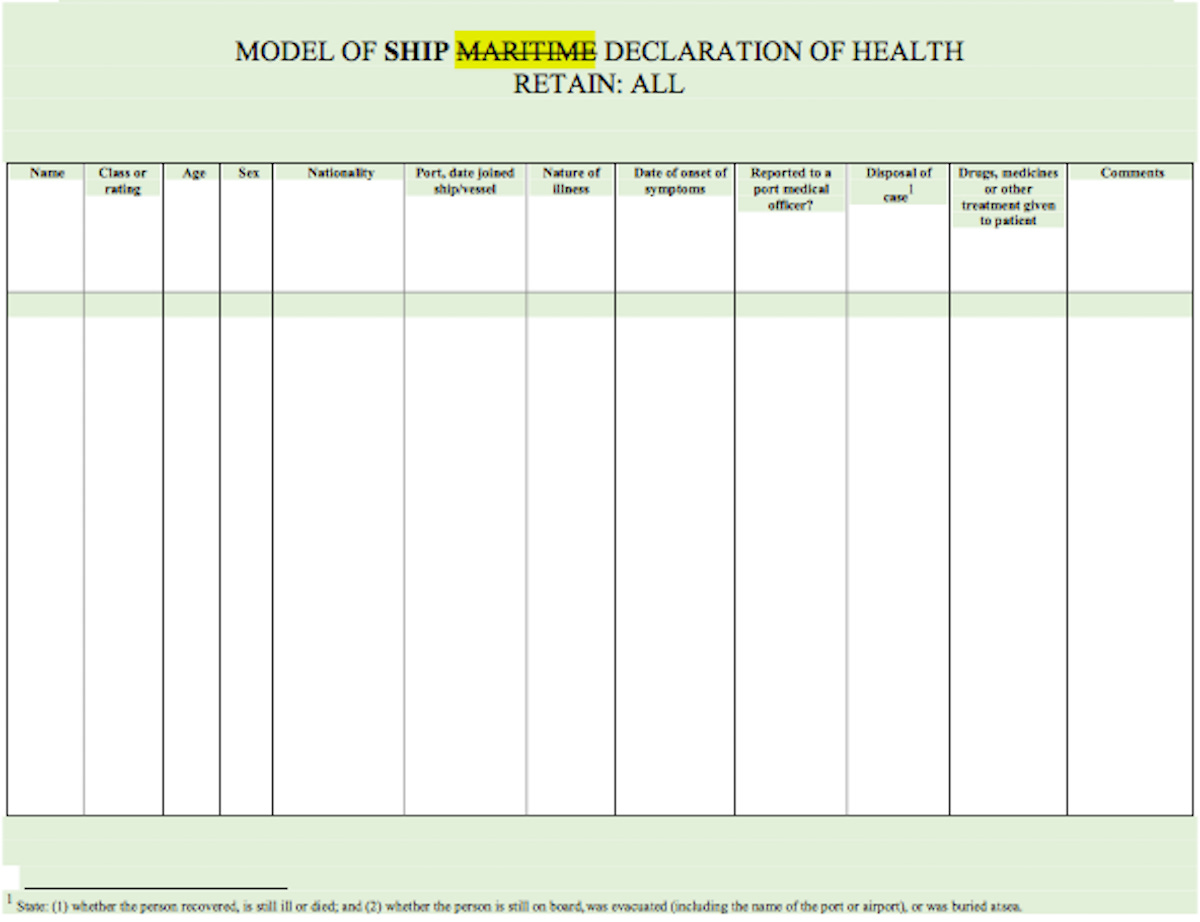
complete and deliver to the competent authority for that port a Ship Maritime Declaration of Health which shall be countersigned by the ship’s surgeon, if one is carried.
2.
The master of a ship, or the ship’s surgeon if one is carried, shall
supply any information required by the competent authority as to health
conditions on board during an international voyage.
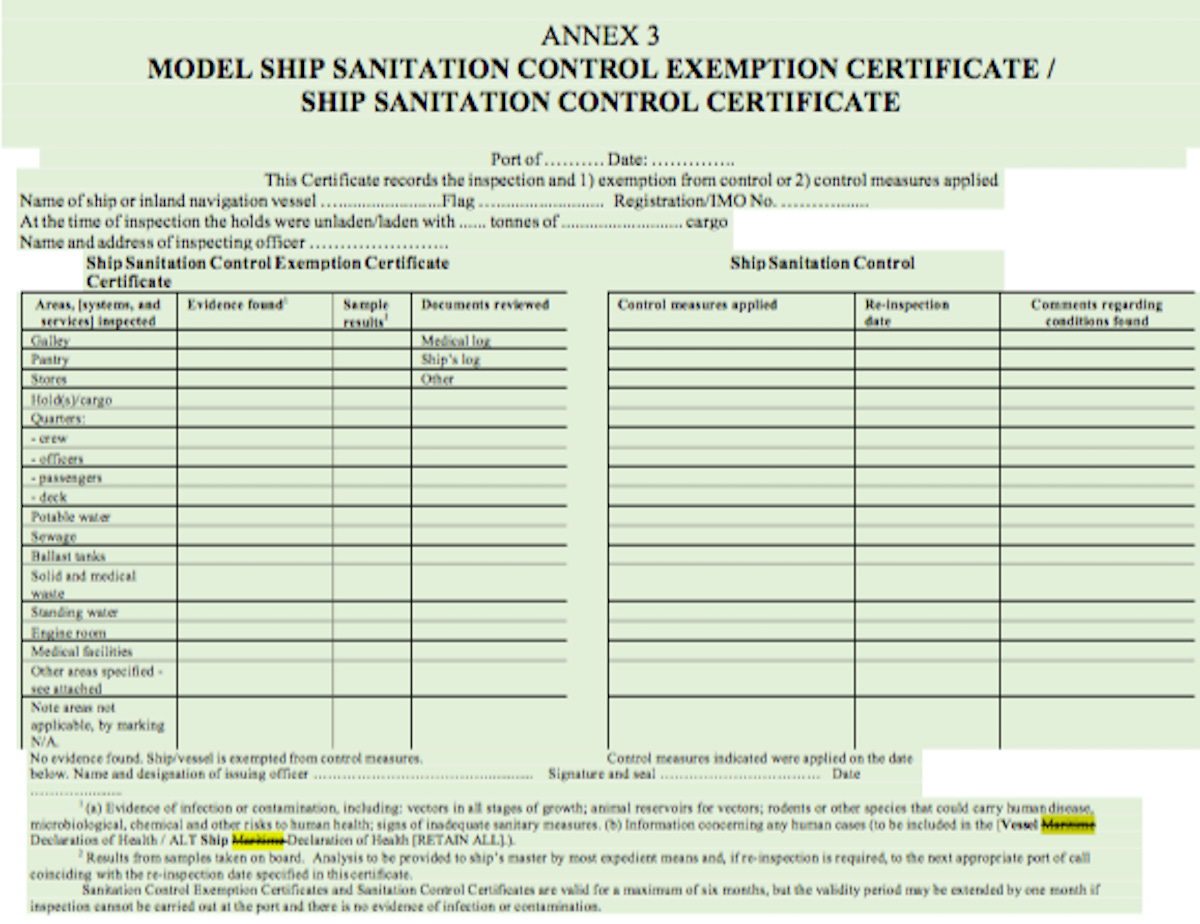
3. A Ship Maritime Declaration of Health shall conform to the model provided in Annex 8.
4. A State Party may decide:
(a) to dispense with the submission of the Ship Maritime Declaration of Health by all arriving ships; or
(b) to require the submission of the Ship Maritime
Declaration of Health under a recommendation concerning ships arriving
from affected areas or to require it from ships which might otherwise
carry infection or contamination.
The State Party shall inform shipping operators or their agents of these requirements.
State
Parties shall take all practicable measures, consistent with relevant
national law and their international obligations, to ensure that
non-State actors operating in their respective jurisdictions comply with
and implement health measures taken pursuant to these Regulations.
3
bis. When implementing additional health measures referred to in
paragraph 1 of this article, States Parties shall take all practicable
measures to avoid interference with and facilitate unhindered and
equitable access to health products required for responding to a public
health risk or a public health emergency of international concern.
1. Upon request of a State Party and/or of WHO, States Parties shall, to the extent possible, undertake to collaborate with, and assist each other, in particular developing countries, to the extent possible, in:
(a) the detection and assessment of, and response to, events as provided under these Regulations,
including by exchanging, samples and genetic sequence data of pathogens
through an equitable and fair ABS system established under the World
Health Organization.
(e)
facilitating unhindered and equitable access to health products,
technologies and know-how through WHO-coordinated mechanisms.
2. WHO shall collaborate with, and assist States Parties, upon request, to the extent possible, in:
(d)
facilitating the exchange of samples and genetic sequence data of
pathogens through an equitable and fair ABS system established under the
World Health Organization.
(e) the formulation of proposed laws and other legal and administrative provisions for the implementation of these Regulations;
(f)
facilitating unhindered and equitable access to health products,
technologies and know-how, through WHO-coordinated mechanisms.
2.
In the event that processing or disclosure of personal data pursuant to
this paragraph would result in public disclosure of such personal data,
the State Party concerned shall inform, if possible prior to such
public disclosure, the State Party that provided the data.
1.
States Parties shall take specific measures to strengthen broader
health systems capacities, such as primary health care and hospital care
facilities, while investing domestically and providing international
assistance for building capacities under this Annex.
New
4. State(s) whose existing/ and or strengthened national structures and
resources are not able to meet the core capacity requirements within
time frame stipulated under para 2, shall be supported by WHO to fill
gaps in critical capacities for surveillance, reporting, notification,
verification, response.
At the local community level and/or primary public health response level (hereinafter the “Local Level”)
Each State Party shall develop, strengthen and maintain,
The capacities:
(c)
to coordinate with and support the local level in preparing for and
responding to public health risks and other events, including in
relation to:
(i) surveillance;
(ii) on-site investigations, including multidisciplinary and /or multisectoral;
(iii) laboratory diagnostics, including referral of samples and genetic sequencing;
(iv) implementation of control measures;
(v) provision of access to health services and relevant health products;
(vi) risk communication, including countering misinformation and disinformation; and
(vi bis) provision of health information system to support emergency operations
(vii) provision of logistical assistance.
5. At the national level
Public health preparedness and response. The capacities:
(a)
to coordinate with and support the local and intermediate levels in
preparing for and responding to public health risks and other events,
including in relation to:
(ii) collaborative / multi-sectoral, multidisciplinary surveillance;
(vii) risk communication, including countering misinformation and disinformation;
B. CORE CAPACITIES REQUIREMENTS FOR DESIGNATED AIRPORTS, PORTS AND
GROUND CROSSINGS
1. At all times
The capacities:
(pre a) to establish surveillance at Points of Entry.
(c) to provide trained Point of Entry workforce personnel for the inspection of conveyances;
1. Conveyance operators shall facilitate:
(c) application of other health measures under these Regulations, including
by providing conveyances with a plan to address situations where there
is evidence of a public health risk on board as well as during
embarkation and disembarkation;
1.
Vaccines or other prophylaxis specified in Annex 7 or recommended under
these Regulations shall be of suitable quality; those vaccines and
prophylaxis designated by WHO shall be prequalified or listed for emergency use by WHO.
3. Certificates under this Annex are valid only if the vaccine or prophylaxis used has been prequalified or listed for emergency use by WHO.
4
bis. In accordance with Article 35, certificates, regardless of the
format in which they have been issued, shall include elements allowing
for the ascertainment of the certificate’s authenticity through
non-digital means. This requirement shall be reviewed by the States
Parties, based on developments, with a view to its modification as
appropriate, at the Eighty-second World Health Assembly and shall
conform with technical guidance and specifications in line with Articles
35 and 45. Without prejudice to the foregoing, certificates may also
include additional elements allowing for the digital ascertainment of
the certificate’s authenticity.
This certificate is valid only if the vaccine or prophylaxis used has been approved prequalified or listed for emergency use by the World Health Organization
The proposal states:
“Certificates under this Annex are valid only if the vaccine or prophylaxis used has been prequalified or listed for emergency use approved by WHO”.
[The words in bold indicate the proposed text addition, and those in strikethrough are proposed for deletion].
The proposal to introduce these phrases came from the United States during the December 2023 meeting.
This
amendment, if adopted, will make it compulsory to use only vaccines or
prophylaxis that are WHO prequalified or listed for emergency use by WHO
to issue vaccine/prophylaxis-related certificates.
https://twn.my/title2/health.info/2024/hi240204.htm
No
amendments have been proposed to Annex 7. The current text from the
International Health Regulations is included here for convenience.
REQUIREMENTS CONCERNING VACCINATION OR PROPHYLAXIS FOR SPECIFIC DISEASES
1.
In addition to any recommendation concerning vaccination or
prophylaxis, the following diseases are those specifically designated
under these Regulations for which proof of vaccination or prophylaxis
may be required for travellers as a condition of entry to a State Party:
Vaccination against yellow fever.
2. Recommendations and requirements for vaccination against yellow fever:
(a) For the purpose of this Annex:
(i) the incubation period of yellow fever is six days;
(ii) yellow fever vaccines approved by WHO provide protection against infection
starting 10 days following the administration of the vaccine;
(iii) this protection continues for the life of the person vaccinated; and
(iv) the validity of a certificate of vaccination against yellow fever shall extend for the
life of the person vaccinated, beginning 10 days after the date of vaccination.
(b)
Vaccination against yellow fever may be required of any traveller
leaving an area where the Organization has determined that a risk of
yellow fever transmission is present.
(c)
If a traveller is in possession of a certificate of vaccination against
yellow fever which is not yet valid, the traveller may be permitted to
depart, but the provisions of paragraph 2(h) of this Annex may be
applied on arrival.
(d)
A traveller in possession of a valid certificate of vaccination against
yellow fever shall not be treated as suspect, even if coming from an
area where the Organization has determined that a risk of yellow fever
transmission is present.
(e) In accordance with paragraph 1 of Annex 6 the yellow fever vaccine used must be approved by the Organization.
(f)
States Parties shall designate specific yellow fever vaccination
centres within their territories in order to ensure the quality and
safety of the procedures and materials employed.
(g)
Every person employed at a point of entry in an area where the
Organization has determined that a risk of yellow fever transmission is
present, and every member of the crew of a conveyance using any such
point of entry, shall be in possession of a valid certificate of
vaccination against yellow fever.
(h)
A State Party, in whose territory vectors of yellow fever are present,
may require a traveller from an area where the Organization has
determined that a risk of yellow fever transmission is present, who is
unable to produce a valid certificate of vaccination against yellow
fever, to be quarantined until the certificate becomes valid, or until a
period of not more than six days, reckoned from the date of last
possible exposure to infection, has elapsed, whichever occurs first.
(i)
Travellers who possess an exemption from yellow fever vaccination,
signed by an authorized medical officer or an authorized health worker,
may nevertheless be allowed entry, subject to the provisions of the
foregoing paragraph of this Annex and to being provided with information
regarding protection from yellow fever vectors. Should the travellers
not be quarantined, they may be required to report any feverish or other
symptoms to the competent authority and be placed under surveillance.
Article 2
Article 3
Article 13A
Article 44A
Article 53A
Article 53 bis-quater
Article 54
Article 54 bis
Annex 10
https://www.who.int/news-room/events/detail/2024/02/05/default-calendar/seventh-meeting-of-the-working-group-on-amendments-to-the-international-health-regulations-(2005)
The
document below is VERY difficult to read. The vast majority of it is
actually text from the current version (2005) of the International
Health Regulations. Only a relatively small portion of the text in the
document below is “bold,” which indicates that it is new (proposed amendment) text. Please realize that this “negotiating text” is subject to change.
https://www.graduateinstitute.ch/sites/internet/files/2024-03/GHC_WGIHR7_Consolidated_Compilation%20of%20Bureau%20text%20proposals_9%20Feb%202024%20%40%2013.00%20CET.pdf
https://apps.who.int/gb/wgihr/e/e_wgihr-8.html
The old system is crumbling, and we must build its replacement quickly.
If
you are fed up with the government, hospital, medical, pharmaceutical,
media, industrial complex and would like to help build a holistic
alternative to the WHO, then feel free to contact me directly anytime.
JamesRoguski.com
JamesRoguski.substack.com/about
JamesRoguski.substack.com/archive
310-619-3055
All content is free to all readers.
All support is deeply appreciated.
CLICK HERE TO DONATE
Share
Leave a comment





































No comments:
Post a Comment