Research Links Mitochondrial Dysfunction to Immune Decline, Opening Paths for Cancer Treatment
- March 14, 2024
Story at-a-glance
- New research shows mitochondrial dysfunction in T cells leads to immune system decline, affecting the body's ability to fight infections and cancer
- The study identifies glycolysis as an inefficient way to produce energy, and the reactive oxygen species (ROS) produced in the process damage T cells
- The findings suggest reversing mitochondrial damage could improve immune response and cancer therapies
- Excess linoleic acid (LA) intake and estrogen dominance are major contributors to mitochondrial dysfunction
- Strategies that will improve your mitochondrial function include lowering your LA intake, reducing stress and taking a niacinamide supplement
Immune system weakening has long been attributed to aging and poor lifestyle choices, but according to an October 2023 study1 in Nature Communications, the key reason for this immune system decline is dysfunctional mitochondria, your cells' powerhouses, particularly the mitochondria found in T cells (a type of immune cell).
When the mitochondria don't work well, the T cells don’t have the energy required to perform their functions, which leads to a decline in immune system function. This in turn, results in an inability to ward off both acute infections and chronic diseases. As reported by Medical Xpress:2
“... In the immune system, chronic infections and the defense against tumors often lead to the phenomenon of T cell exhaustion: In this process, the T lymphocytes gradually lose their function, which impairs their responses against cancer and infections ...
This research has now shown that the exhaustion process is significantly influenced by ... the mitochondria. When mitochondrial respiration fails, a cascade of reactions is triggered, culminating in the genetic and metabolic reprogramming of T cells, a process that drives their functional exhaustion.”
The good news, which was confirmed by the featured study, is that this decline can be reversed with treatments that target mitochondrial function.
Poor Mitochondrial Function Can Lead to T Cell Exhaustion
In simple terms, when your body fights an infection, immune cells called CD8+ T cells transform into cytotoxic T lymphocytes (CTLs) to destroy the infected cells. This transformation requires changes in gene expression, cell structure and energy use.
However, in long-lasting infections or cancer, the T cells can become worn out or "exhausted," losing their effectiveness. This exhaustion is related to energy problems within the cells, particularly in the mitochondria. Researchers are now exploring how fixing these energy problems can rejuvenate exhausted T cells, thereby improving cancer treatments. Bioenergetic researcher Georgi Dinkov comments on these findings:3
“So far, the decline in immune function seen in aging had been explained with the simplistic ‘wear and tear’ concept, and when immunodeficiency occurs in younger people it is ascribed either to genetic vulnerability or lifestyle choices such as alcohol/drug consumption.
In other words, to this day medicine does not seem to have a good grip on why immune function fails in aging and disease, and what (if anything) can be done to prevent that.
The study ... demonstrates that the direct cause of immune decline is rather simple — decline in mitochondrial function (OXPHOS). When T-cells (immune cells produced by the thymus) have dysfunctional mitochondria, they have to rely exclusively on glycolysis for energy production.
Glycolytic production of energy is insufficient to support proper T-cell differentiation and activity, and in fact can lead to T-cell damage or even death due to the high amount of reactive oxygen species (ROS) produced when glycolysis is the main mode of energy production.
The study also demonstrated that such mitochondrial dysfunction is a necessary and sufficient condition for immune decline to occur (aka T-cell ‘exhaustion’) and that the decline was reversible when mitochondrial function was restored pharmacologically.”
Glucose Metabolism 101
So, what is “glycolytic production of energy” and why is it so detrimental? All dietary carbohydrates are digested and broken down into glucose, a type of sugar. Glucose, in turn, can be metabolized (burned) for fuel using two different pathways, as illustrated below.
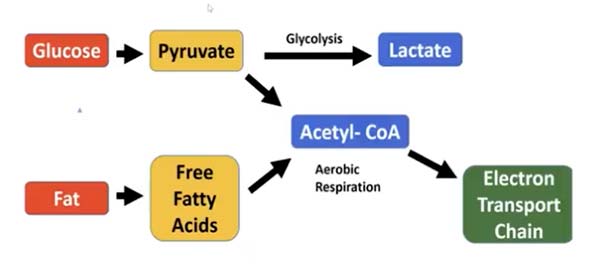
First, the glucose is metabolized into pyruvate. The pyruvate can then either enter the glycolysis pathway in the cytoplasm of the cell and produce lactate, or it can be converted into acetyl-CoA and shuttled to the mitochondrial electron transport chain.
Cancer cells are notorious for using the glycolysis pathway — the same pathway glucose goes through when your glucose metabolism is impaired in mitochondria. Basically, this is the pathway your body uses whenever it reaches its limit to how much ATP can be produced in the mitochondria (which is the most efficient and least damaging way to produce energy).

Save This Article for Later - Get the PDF Now
Download PDFThe Downstream Hazards of Glycolysis
While the glycolysis pathway is wonderful when you need quick fuel, if this is the primary way you burn glucose, then you are in a constant state of activating stress hormones and promoting insulin resistance and diabetes, which in turn creates loads of lactate as a waste product instead of healthy carbon dioxide (CO2) and metabolic water.
Lactate increases reductive stress, which causes reverse electron flow in the mitochondria and increases the ROS to 3% to 4%, which is 30 to 40 times more than when glucose is burned in the mitochondria. This elevated ROS production is what causes T cell damage and death.
What’s more, glycolysis generates only two ATP for every molecule of glucose, which is 95% less energy than would be generated if the glucose was metabolized in your mitochondria.
The Devil’s in the Details
Now, you’ve probably heard that sugar promotes cancer, because cancer cells preferentially use glycolysis. However, it’s a mistake to think that all glucose uses the glycolysis pathway. As illustrated above, glucose can also be burned in the electron transport chain of the mitochondria, which is the most efficient way to produce energy.
So, when it comes to the “sugar fuels cancer” issue, it’s important to make a distinction between the sources of the carbs. While it is technically accurate to call all carbs sugar, there is a radical difference in the source of the carbs — ripe whole fruits versus starches, for example, and whole fruits versus refined processed sugar (ex: table sugar and high fructose corn syrup).
Refined sugars, as well as many starches, are a common cause of endotoxin production in your gut, which destroys mitochondrial function and results in cancer metabolism, whereas the fructose present in whole foods does not typically result in the production of endotoxin.
This is one of the primary differences between refined sugar and fructose from ripe fruit and helps explain why refined sugars fuel cancer while natural fructose does not. So, to be clear, it’s not sugar that is driving the cancer process per se. It’s really rooted in mitochondrial dysfunction, and fatty acid oxidation (the metabolism of fats instead of glucose) is part of what causes that dysfunction.
You Want to Burn Glucose in Your Mitochondria
For a long time, I believed fats burned “cleaner” than carbs — that’s one of the “selling points” for keto — but I’ve since realized we had it backward. Glucose, when burned in the mitochondria, actually burns far cleaner than fat.
So, it’s important to get your macronutrient ratios right, because if the glucose you eat is constantly shuttled into glycolysis, you’re fueling cancer. At the same time, the fat you consume ends up in fat storage rather than being used up for fuel.
Ultimately, you want to burn glucose in your mitochondria, and the way you ensure that is by keeping your dietary fat intake below 35% of your total calories. The reason for this is because when fat intake is too high, glucose gets shuttled into glycolysis. For a more in-depth explanation of this metabolic switch, see “Understanding the Randle Cycle.”
If you’re insulin resistant, which means you’re metabolically inflexible, that threshold may be closer to 20% or even 10%. So, if you’re insulin resistant, you’ll want to significantly lower your fat intake until your insulin resistance is resolved. Then you can increase it to 30%.
Dysfunctional T Cells and Cancer Cells Use Glycolysis
The reason cancer cells use the glycolysis pathway is because they have severely dysfunctional mitochondria. The mitochondria are so damaged, they cannot burn glucose. As a result, the cancer cells must rely on the backup system, glycolysis, to survive. This is what the Warburg Effect is all about.
Likewise, when the mitochondria inside T cells become dysfunctional, the T cells are forced to rely on glycolysis for energy production, which is what causes immune system weakening and failure.
As mentioned in the featured study, T cell exhaustion is a feature of cancer, which makes sense when you consider that it’s all tied to mitochondrial dysfunction. The cancer starts because the mitochondria in cells are severely damaged, and as the disease progresses, the mitochondria in the T cells begin to fail as well.
Since mitochondrial dysfunction is at the heart of it all, the most effective strategy is to use metabolic therapies that address why the cells are unable to oxidize (burn) sugar in the mitochondria. Once you fix the mitochondria so that they can generate sufficient energy again, then the cancer will typically regress and immune function will be restored, as they no longer need to rely on glycolysis.
What Causes Mitochondrial Dysfunction?
There are four primary contributors to mitochondrial dysfunction:
- Excess linoleic acid (LA) intake
- Estrogen dominance
- Electromagnetic field (EMF) exposure
- Endotoxin — Refined sugars and many starches are more likely to cause gut dysbiosis that leads to the production of endotoxin. This endotoxin is one of the factors that destroys mitochondrial function, resulting in the Warburg Effect (cancer metabolism), where glucose is burned through glycolysis
These all play major roles, but excess LA and estrogen dominance, I believe, are the leading contributors to mitochondrial dysfunction. This is largely because LA and estrogen negatively impact your body in similar ways. They both:
- Increase free radicals that cause oxidative stress and damage your mitochondria’s ability to produce energy.
- Increase calcium intake inside the cell that causes an increase in nitric oxide and superoxide that increases peroxynitrite that also increases oxidative stress.
- Cause an increase in intracellular water causing your body to retain water.
- Slow down your metabolic rate and suppress your thyroid gland.
Nearly everyone in the developing world has 10 times the amount of LA in their tissues than their ancestors of 100 years ago had. This polyunsaturated fat (PUFA) is very susceptible to oxidative damage, and produces free radicals like reactive aldehydes in your body that destroy your mitochondria.
These toxic metabolites of LA create enormous amounts of reductive stress as a result of electrons building up in the ETC and blocking the forward movement of electrons to complex IV and V to create ATP. And, because LA is embedded in the inner mitochondrial membrane, it gets damaged and leaks protons that normally build up in the inner mitochondrial space.
This proton gradient is responsible for driving the nano motor in complex V to create ATP. Both processes combine to shut down and ultimately prematurely destroy the mitochondria. Also, when you eat starches, they can end up feeding endotoxin-producing bacteria in your intestine, and endotoxin is a potent mitochondrial poison.
Solutions
In closing then, some of the key solutions, if you want to improve or restore your mitochondrial function, would be to:
- Lower your LA intake as low as possible by avoiding processed foods, seed oils, chicken, pork, seeds and nuts.
- Make sure you’re eating healthy carbs such as ripe fruit, raw honey and maple syrup.
- Decrease lactate production and increase carbon dioxide, as they have opposing effects.4 You can learn more about this in “The Biology of Carbon Dioxide.”
- Reduce your stress, as chronic stress promotes cortisol release, which is a potent suppressor of mitochondrial function and biogenesis. Progesterone can be quite helpful here, as it’s a potent cortisol blocker. You can learn more about this in “What You Need to Know About Estrogen and Serotonin.”
- Take supplemental niacinamide, as your mitochondria cannot make energy without it. I recommend taking 50 mg of niacinamide three times a day.
No comments:
Post a Comment