Regulatory simulations at home and abroad: statutory and regulatory definitions for drugs, biological products, and biosimilars.Information to support further reporting on regulation and non-regulation of biological product manufacturing, sample testing, lot-release, use.American Domestic Bioterrorism Program Related Bailiwick reporting and analysis
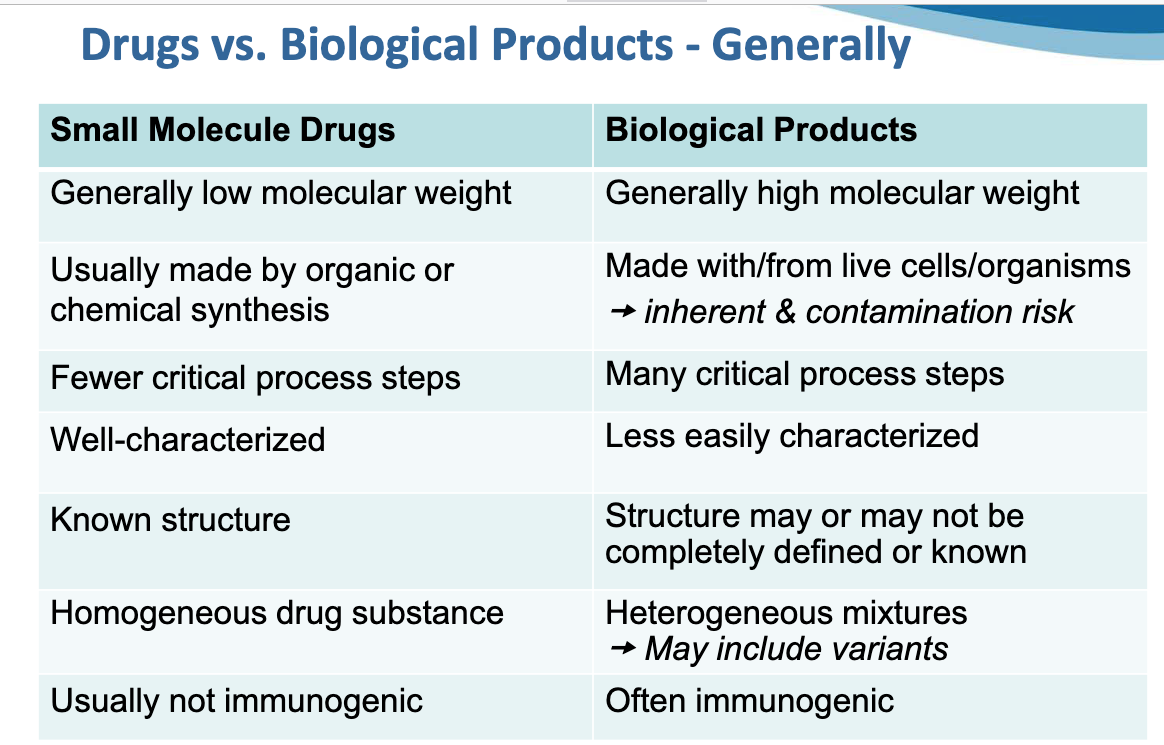
This overview is focused on the development of biological product definitions, statutes and regulations since July 1944, when Congress established the Regulation of biological products program under the Public Health Service Act at Section 351, codified at 42 US Code 262.Biological product manufacturing regulations may be found at 21 CFR Subchapter F, Parts 600 to 680 and related sections of the Code of Federal Regulations as they have developed, especially since 1973. Key points: Chemical drugs are produced by quantifiable, predictable, controllable chemical and physical manufacturing processes involving the breaking and forming of chemical bonds; they tend to have smaller molecular structures than biological products. Biological products are produced by non-quantifiable, unpredictable, uncontrollable biological processes, such as replication and division within living cells and organisms, with widely variable effects on other living organisms when introduced into a recipient. Biological products tend to have larger molecular structures than chemical drugs. Chemical drugs manufactured using predictable, measurable chemical reactions can be produced and assessed for compliance with purity, potency, activity, safety and efficacy standards. Biological products manufactured using biological processes cannot. Statutory definitions of "drug" June 30, 1906 - Pure Food and Drug Act, PL 59-384, 34 Stat. 768. Through the Pure Food and Drug Act, Congress prohibited adulteration and misbranding of drugs, and established civil and criminal penalties for manufacturers producing and distributing adulterated and misbranded drugs. Congress delegated authority to promulgate and enforce rules and regulations to the Secretary of the Treasury, Secretary of Agriculture, Secretary of Commerce and Labor, and US district attorneys, including "collection and examination of specimens." Biological products, including "viruses, therapeutic serums, toxins, antitoxins, or analogous products" had been listed in a different Congressional act, signed in 1902 (see below) and were not covered by the Pure Food and Drug Act. Congress defined "drug" to include
June 25, 1938 - Federal Food Drug and Cosmetics Act, PL 75-717, 52 Stat. 1041. Through the Food Drug and Cosmetics Act (FDCA), Congress repealed and replaced the 1906 Pure Food and Drug Act, and codified federal food and drug regulation at 21 USC Chapter 9, Sections 301 et seq. Congress defined drug at 21 USC 321(g).
Current FDCA Section 201/ 21 USC 321(g)(1)
Drugs@FDA Glossary of Terms, last updated Nov. 14, 2017
Statutory and regulatory definitions of biologics, biological products and biosimilars July 1, 1902 - Biologics Control Act or Virus-Toxin Act (32 Stat. 728). Through the Biologics Control Act, Congress regulated "sale of and interstate traffic" in viruses, serums, toxins and analogous products, and delegated authority to promulgate and enforce rules and regulations to the Secretary of Treasury, in consultation with the Surgeon-Generals of the Army, Navy and Marine-Hospital Service.
March 4, 1913 - Virus-Serum Toxin Act, 37 Stat. 832. Through the Virus-Serum Toxin Act, Congress regulated preparation and sale of "virus, serum, toxin, or analogous product intended for use in the treatment of domestic animals," and delegated authority to promulgate and enforce rules and regulations to the Secretary of Agriculture.
July 1, 1944 - Public Health Service Act (PHSA) Section 351, Regulation of biological products. PL 78-410, 58 Stat. 702. Through the PHSA, Congress codified regulation and licensing of biological product manufacturing at 42 USC 262. PHSA Section 351, Regulation of biological products (1944):
Notes Congress did not place regulation of biological products under the Food Drug and Cosmetics Act, or under the Food and Drug Administration. Instead, Congress placed regulation of biological products under the control of the Public Health Service, which is a branch of the US military. Congress provided a list of biological product categories to be regulated under 42 USC 262, but did not provide legal definitions of the specific products to be regulated, instead describing them generally as products "applicable to the prevention, treatment or cure of diseases or injuries of man." Between 1937 and 1972, biological product regulation was housed in the National Institute of Health Division of Biologics Standards. In 1972, biological product regulation was moved to the FDA Bureau of Biologics, now called the Center for Biologics Evaluation and Research, or CBER. Oct. 30, 1970 - Heart Disease, Cancer, Stroke, and Kidney Disease Amendments of 1970. PL 91-515, 84 Stat. 1297. Congress added vaccine to the list of biological products subject to manufacturing regulation under the Public Health Service Act.
As of 1970, biological products listed by Congress as subject to federal manufacturing regulation under 42 USC 262 included:
Nov. 20, 1973 - Department of Health, Education and Welfare, Food and Drug Administration, Notice of Reorganization and Republication, 38 Federal Register 32048 Through this Federal Register notice, FDA Acting Associate Commissioner for Compliance William F. Randolph announced the consolidation and re-publication of federal regulations governing biological product manufacturing. Sections included 21 CFR 600, Biological Products: General; 21 CFR 601, Licensing; 21 CFR 610, General Biological Products Standards; 21 CFR 620, Additional Standards for Bacterial Products; 21 CFR 630, Additional Standards for Viral Vaccines; 21 CFR 640, Additional Standards for Human Blood and Blood Products; and three other sections. FDA defined several terms at 21 CFR 600.3, but did not define the term vaccine.
Nov. 18, 1997 – National Defense Authorization Act FY1998, PL 105-85, 111 Stat. 1915. Congress amended 50 USC 1520a, Restrictions on the use of human subjects for testing of chemical or biological agents. In the wake of the Gulf War (1990-1991), during which DoD forced soldiers to submit to batteries of vaccines and toxic exposures in theatre, including burn pits, Congress defined "biological agents."
Note The November 1997 NDAA section on biological agents is one of the main Congressional two-part maneuvers through which Congress appeared to be terminating illegal chemical and biological warfare programs, but actually just moved, renamed and expanded the same programs as public health emergency-medical countermeasures programs.
March 23, 2010 - Biologics Price Competition Act, Title VII, Subtitle A of Patient Protection and Affordable Care Act, PL 111-148, 124 Stat. 814-815. Through the BPCA, Congress added "protein (except any chemically synthesized polypeptide)" and added a new category of biosimilars to the list of biological products subject to regulation under 42 USC 262.
As of 2010, the list of biological products listed by Congress as subject to regulation under 42 USC 262 included:
Dec. 20, 2019 - Further Consolidated Appropriations Act, 2020, PL 116-94, 133 STAT. 3127 Congress removed "(except any chemically synthesized polypeptide)" — which had been added, with "protein" in 2010 — from the biological products definition.
As of December 2019, biological products listed by Congress as subject to regulation under 42 USC 262 include:
FDA "What is a biological product?" (Undated, no author listed)
Statutory and regulatory definitions of "vaccine" Congress established the federal biological products licensing and regulation program in 1944. Vaccines were added to the list of regulated, licensed biological products by Congressional statute in 1970, and regulatory, licensing functions were transferred from NIH to FDA in 1972; Congress did not define the term vaccine. FDA regulations covering biological product manufacturing, including vaccines, were consolidated and re-published in 1973, and have been amended extensively since; FDA did not define the term vaccine. In 1976, Congress authorized and funded a nationwide vaccination campaign including liability exemption for manufacturers, for swine flu, (National Swine Flu Immunization Act, PL 94-380, 90 Stat. 1113) without defining the term vaccine. In 1986, Congress authorized and funded a nationwide child vaccination program, including liability exemption for manufacturers and establishment of the Vaccine Injury Compensation Program, (National Childhood Vaccine Injury Act, PL 99-660, 100 Stat 3755, codified at 42 USC 300aa-1 to 34), without defining the term vaccine. Vaccine has not been defined by Congress through amendments to the Food Drug and Cosmetics Act (FDCA), or to the Public Health Service Act (PHSA), and the term has not been defined by the FDA through regulations published in the Federal Register. In 1987, Congress provided a statutory definition of vaccine through in the Internal Revenue Code, 26 USC 4132. The "Certain vaccines" provision authorized collection of excise tax by the Treasury Secretary, from manufacturers, per dose of vaccine sold. The list of taxable vaccines has been expanded since 1987. 26 USC 4132a(2) Vaccine.
26 USC 4132a(1) Taxable vaccine
Federal public health officials have also published definitions of the term vaccine that have not been established by statute or regulation. Some are dictionary definitions or medical and scientific definitions, including the revised definition promulgated by CDC in September 2021, replacing "a product that stimulates a person's immune system to produce immunity to a specific disease, protecting the person from that disease," to "a preparation that is used to stimulate the body's immune response against diseases." CDC-Advisory Committee on Immunization Practices Glossary (ACIP) currently defines vaccine:
CDC Vaccines and Immunizations Glossary defines vaccine:
Brief Analysis There are many terms for, and/or related to, biological products currently in use. Statutes, regulations, FDA guidance documents, World Health Organization documents, Mutual Recognition Agreements, and other records include allergen; allergenic product; antitoxin; antigen; biopharmaceutical; biosimilar biological product; biotechnology product; biotechnology; blood, blood component, or derivative; cell therapies; emerging technology in the context of the pharmaceutical and related industries; first interchangeable biosimilar biological product; gene therapies; immunogen; intentionally altered genomic DNA; monoclonal antibody; plasma-derived pharmaceutical; plasma-derived product; plasmid; polypeptide; protein; recombinant protein; reference product; somatic cell therapy; synthetic biological product; therapeutic biotechnology-derived biological product; therapeutic recombinant DNA-derived product; therapeutic serum; vaccine; virus; toxin; and more. Many of the documents acknowledge the extent to which biological product manufacturing cannot be standardized, such that product purity is an impossible regulatory standard for any biological product to achieve. Manufacturing quality for a given package of biological material can, at best, contain a percentage of product assayed to be in conformity with contents as described on the label, at the moment of sample testing. Even if products meet limited, fractional purity standards at the moment of sample testing, the contents of each package are subject to further changes over time due to metabolic processes and byproducts, sedimentation, mixing, temperature changes, degradation and other factors, because the contents are comprised of living, dynamic and therefore non-stable components. After entering the body of each recipient, each biological product undergoes additional unpredictable, widely variant changes as the components interact with the living organism through billions of biological events. Some documents
All content is free to all readers. All support — reading, sharing and financial — is deeply appreciated. |

No comments:
Post a Comment