I've been reading Covid-times case law related to:
1) Constitutional separation of powers between the three distinct branches of the federal government — executive (President and administrative Cabinet secretaries and agencies); legislative (Congress); and judicial (federal district courts, circuit courts of appeals and Supreme Court); and
2) Constitutional federalism, or reservation of powers — powers "not delegated" by the States and the people to the federal government and powers not "prohibited by" the Constitution — to the States, under Amendment 10.
The powers not delegated to the United States by the Constitution, nor prohibited by it to the States, are reserved to the States respectively, or to the people.
There are two main ways that the monsters working in and through the United Nations World Health Organization preemptively hobbled the US Constitution as embodied in American governing
institutions, that would have interfered with the Covid-19 sequence of orchestrated lies and stopped the ongoing mass murder program.One mechanism for the kneecapping of the Constitution is through the laws passed by Congress and signed by US presidents. More on those statutory mechanisms below.
The other main mechanism is through federal court decisions that have interpreted the Constitution expansively with regard to exercise of federal power, and narrowly with regard to exercise of State power.
Through his May 29, 2020 opinion in South Bay Pentecostal Church v. Gavin Newsom, et al, SCOTUS Chief Justice John Roberts issued a stand-down order to block all federal courts from reviewing federal and state exercise of executive and legislative power for constitutional soundness.
Justice Roberts cited a 1985 case, Garcia v. San Antonio Metropolitan Transit Authority et al, to support his argument:
…Where those broad limits [on latitude to act for "the safety and health of the people"] are not exceeded, they should not be subject to second-guessing by an “unelected federal judiciary,” which lacks the background, competence, and expertise to assess public health and is not accountable to the people…
More on South Bay Pentecostal v. Newsom and Garcia v. SAMTA to come.
Bailiwick reporting and short analyses of South Bay Pentecostal v. Newsom and Congressional abdication/executive usurpation of Constitutional authority at footnote.¹ [insert footnote]
On the statutory side, the Constitutional damage was mostly inflicted at 42 USC 247d-6d(b)(7), (8) and (9): provisions added to the Public Health Service Act of 1944 in 2005 through the PREP Act.
For background:
The "public health emergency" section (PHSA 319, 42 USC 247d) was added to the PHSA in July 1983. The 1983 Congressional act introduced the category of "public health emergency" to the collection of national circumstances (such as state of war) authorizing overrides of constitutional law, civil tort law and criminal law.
42 USC 247d = PHSA 319:
PUBLIC HEALTH EMERGENCIES.
(a) EMERGENCIES.—If the Secretary determines, after consultation with the Director of the National Institutes of Health, the Administrator of the Alcohol, Drug Abuse, and Mental Health Administration, the Commissioner of the Food and Drug Administration, or the Director of the Centers for Disease Control, that—
(1) a disease or disorder presents a public health emergency; or
(2) a public health emergency, including significant outbreaks of infectious diseases or bioterrorist attacks, otherwise exists,
the Secretary may take such action as may be appropriate to respond to the public health emergency, including making grants and entering into contracts and conducting and supporting investigations into the cause, treatment, or prevention of a disease or disorder described in paragraph (1).
The act was very short, just over one page, and the second part appropriated $30 million for a Public Health Emergencies Fund: the slush fund of money to support HHS Secretary "action."
The only oversight provision in the act was a requirement that the HHS Secretary provide annual, retrospective reports to the House Energy and Commerce Committee and the Senate Labor and Human Resources Committee.
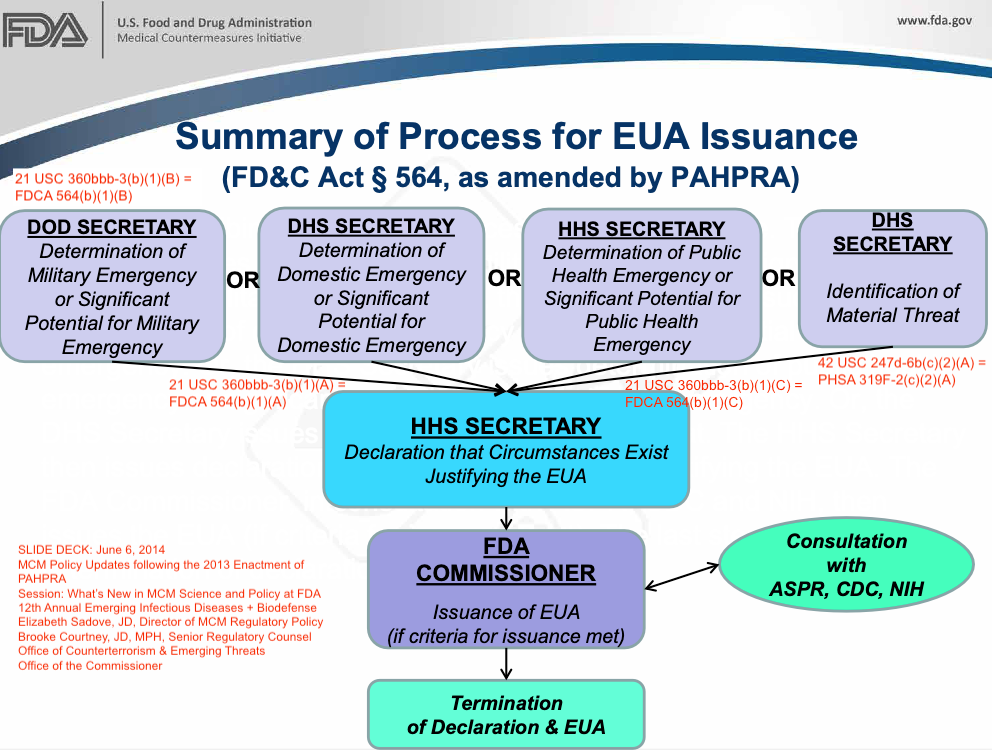
In 2013, the HHS Secretary authority to make a "determination" about the existence of a public health emergency was also added to the Food Drug and Cosmetics Act (FDCA Section 564 = 21 USC 360bbb-3) through the Pandemic and All-Hazards Preparedness Reauthorization Act (PAHPRA Act), to connect the event "determination" to the HHS power to deploy "Emergency Use Authorized" products and platforms:
21 USC 360bbb-3(b) = FDCA 564(b).
DECLARATION OF EMERGENCY OR THREAT JUSTIFYING EMERGENCY AUTHORIZED USE.—
(1) IN GENERAL.—The [HHS] Secretary may make a declaration that the circumstances exist justifying the authorization under this subsection for a product on the basis of—
(A) a determination by the Secretary of Homeland Security that there is a domestic emergency, or a significant potential for a domestic emergency, involving a heightened risk of attack with a biological, chemical, radiological, or nuclear agent or agents;
(B) a determination by the Secretary of Defense that there is a military emergency, or a significant potential for a military emergency, involving a heightened risk to United States military forces, including personnel operating under the authority of title 10 or title 50, United States Code, of attack with—
(i) a biological, chemical, radiological, or nuclear agent or agents; or
(ii) an agent or agents that may cause, or are otherwise associated with, an imminently life-threatening and specific risk to United States military forces;
(C) a determination by the [HHS] Secretary that there is a public health emergency, or a significant potential for a public health emergency, that affects, or has a significant potential to affect, national security or the health and security of United States citizens living abroad, and that involves a biological, chemical, radiological, or nuclear agent or agents, or a disease or condition that may be attributable to such agent or agents; or
(D) the identification of a material threat pursuant to section 319F–2 of the Public Health Service Act sufficient to affect national security or the health and security of United States citizens living abroad. [42 USC 247d-6b(c)(2)(A)]
Then-HHS Secretary Alex Azar invoked and exercised his power under 21 USC 360bbb-3(b)(1)(C), in his Feb. 4, 2020 Notice of Determination of Public Health Emergency and Declaration that "circumstances exist justifying the authorization of emergency use of in vitro diagnostics," a reference to PCR and other Covid-19 testing products. (85 FR 7316).
Also effective Feb. 4, 2020, (signed March 10, 2020, published March 17, 2020, 85 FR 15198), as amended (signed June 4, 2020, published June 8, 2020, 85 FR 35100) was Azar's Declaration Under the Public Readiness and Emergency Preparedness [PREP] Act for Medical Countermeasures Against Covid-19, invoking and exercising HHS Secretary power to exempt all the people involved in medical countermeasures [biochemical weapons] development, manufacture, distribution and use, from legal liability for their actions (PHSA 319F-3 = 42 USC 247d-6d), and to divert all injury and death claimants into the dead-end Countermeasures Injury Compensation Program (CICP), (PHSA 319F-4 = 42 USC 247d-6e.)
The PREP Act, passed on Dec. 30, 2005, is where Congress and President George W. Bush made more explicit, the intentional dismantling of the constitutional principles of both separation of powers and federalism (reservation of powers to the states).
Congress and President Bush stripped Congress of its authority to oversee or terminate emergency declarations and determinations made unilaterally by the HHS Secretary; stripped federal courts of their authority to review or nullify declarations and determinations; and stripped states, tribes, and municipalities (political subdivisions of states) of their authority to apply their own constitutions and laws to the declarations, determinations, products and uses directed by the HHS Secretary as part of the federal executive branch.
42 USC 247d-6d(b)(1) = PHSA 319F-3(b)(1)
DECLARATION BY SECRETARY. (1) AUTHORITY TO ISSUE DECLARATION.—Subject to paragraph (2) [list of declaration contents], if the Secretary makes a determination that a disease or other health condition or other threat to health constitutes a public health emergency, or that there is a credible risk that the disease, condition, or threat may in the future constitute such an emergency, the Secretary may make a declaration, through publication in the Federal Register, recommending, under conditions as the Secretary may specify, the manufacture, testing, development, distribution, administration, or use of one or more covered countermeasures, and stating that subsection (a) is in effect with respect to the activities so recommended.
42 USC 247d-6d(b)(7) = PHSA 319F-3(b)(7)
JUDICIAL REVIEW. No court of the United States, or of any State, shall have subject matter jurisdiction to review, whether by mandamus or otherwise, any action by the Secretary under this subsection.
42 USC 247d-6d (b)(8) = PHSA 319F-3(b)(8)
PREEMPTION OF STATE LAW. During the effective period of a declaration under subsection (b), or at any time with respect to conduct undertaken in accordance with such declaration, no State or political subdivision of a State may establish, enforce, or continue in effect with respect to a covered countermeasure any provision of law or legal requirement that—
(A) is different from, or is in conflict with, any requirement applicable under this section; and
(B) relates to the design, development, clinical testing or investigation, formulation, manufacture, distribution, sale, donation, purchase, marketing, promotion, packaging, labeling, licensing, use, any other aspect of safety or efficacy, or the prescribing, dispensing, or administration by qualified persons of the covered countermeasure, or to any matter included in a requirement applicable to the covered countermeasure under this section or any other provision of this Act, or under the Federal Food, Drug, and Cosmetic Act.
42 USC 247d-6d (b)(9) = PHSA 319F-3(b)(9)
REPORT TO CONGRESS. Within 30 days after making a declaration under paragraph (1), the Secretary shall submit to the appropriate committees of the Congress a report that provides an explanation of the reasons for issuing the declaration and the reasons underlying the determinations of the Secretary with respect to paragraph (2). Within 30 days after making an amendment under paragraph (4), the Secretary shall submit to such committees a report that provides the reasons underlying the determination of the Secretary to make the amendment.
Related Bailiwick reporting and analysis:
June 9, 2021 - Courts, judges, constitutions, lawsuits and evidence are no longer a plausible bulwark against tyranny. “A couple of months ago, Stacey Rudin and Michael Senger commented on Twitter about Chief Justice John Roberts comment in a California case, [South Bay Pentecostal v. Newsom] that appointed judges should not second guess elected executives and legislatures, on the “reasoning” because the elected representatives are closer to the people. Senger and Rudin speculated that Justice Roberts was thereby signaling all of the federal and state courts to quietly dismiss or stall civil liberties cases, to protect the lockdowns from judicial review and protect the lockdown government officials from effective accountability.”
April 7, 2022 - Responding to Steve Kirsch, James Roguski and others. World War Biochemistry has been underway for decades, key battle won by World Health Organization silently in January 2020.
April 7, 2022 - Re: “judicially-unreviewable.”
April 22, 2022 - Administrative Procedures Act v. Public Health Service Act. USDC Middle Florida ruling in Health Freedom Defense Fund v. Biden opens window into key separation of powers issue of the American biomedical police state established Jan. 31, 2020.
June 7, 2022 - On why and how globalists, allied with communists, are fomenting federalist conflicts in America. They aim to block American Christians and Constitutionalists from working together to protect individual human liberty to freely discern and work the will of God. — “The federal courts have been offline for Constitutional issues related to government’s Covid mitigation measures since May 2020, when SCOTUS Chief Justice John Roberts used his opinion in South Bay Pentecostal v. Newsom to direct federal judges to refuse to review executive and legislative acts undertaken in the context of the declared public emergency. The federal judges have complied, including multiple instances of SCOTUS justices refusing appeals of constitutional cases without explanation.”
Sept. 5, 2023 - On Catholic subsidiarity as the counterweight to Satanic secular-materialist centralization of power.
All content is free to all readers.
All support — reading, sharing and financial — is deeply appreciated.

No comments:
Post a Comment