29,031 Deaths, 240,022 Serious Injuries Reported to VAERS, as CDC Admits Not Monitoring System for Safety Signals
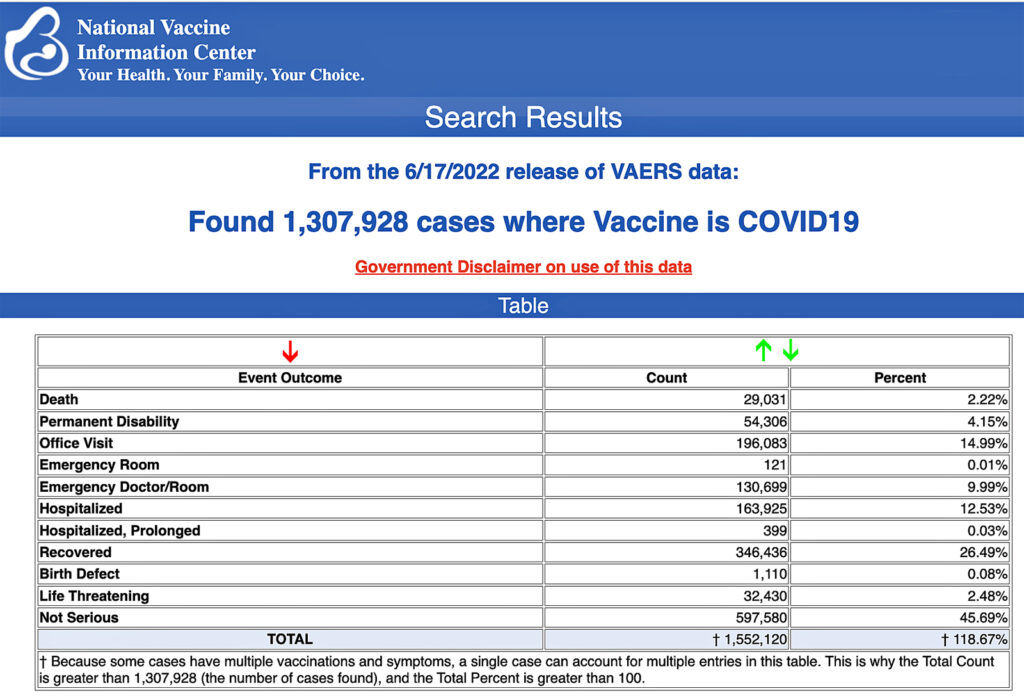
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,307,928 reports of adverse events from all age groups following COVID-19 vaccines, including 29,031 deaths and 240,022 serious injuries between Dec. 14, 2020, and June 17, 2022.
Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,307,928 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and June 17, 2022, to the Vaccine Adverse Event Reporting System (VAERS). That’s an increase of 6,572 adverse events over the previous week.
VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 29,031 reports of deaths — an increase of 172 over the previous week — and 240,022 serious injuries, including deaths, during the same time period — up 1,610 compared with the previous week.
Of the 29,031 reported deaths, 18,814 cases are attributed to Pfizer’s COVID-19 vaccine, 7,627 cases to Moderna and 2,525 cases to Johnson & Johnson (J&J).
Excluding “foreign reports” to VAERS, 835,063 adverse events, including 13,388 deaths and 84,542 serious injuries, were reported in the U.S. between Dec. 14, 2020, and June 17, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 13,388 U.S. deaths reported as of June 17, 16% occurred within 24 hours of vaccination, 20% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 592 million COVID-19 vaccine doses had been administered as of June 16, including 349 million doses of Pfizer, 223 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to June 17, 2022, for 6-month-olds to 5-year-olds show:
- 1,757 adverse events, including 64 cases rated as serious and 3 reported deaths.
- 4 reports of myocarditis and pericarditis (heart inflammation).
- The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department.
- 13 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to June 17, 2022, for 5- to 11-year-olds show:
- 11,534 adverse events, including 298 rated as serious and 6 reported deaths.
The most recent reported death (VAERS I.D. 2315376) occurred in a 9-year-old female from Florida who died 172 days after receiving Pfizer’s vaccine. She was diagnosed with COVID-19 on May 28, 2022, and treated with various drugs, including Remdesivir. She was found unresponsive at home on June 3, and was declared brain dead.
- 22 reports of myocarditis and pericarditis.
The Defender has noticed over previous weeks that reports of myocarditis and pericarditis have been removed by the CDC from the VAERS system in this age group. No explanation was provided.
- 44 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to June 17, 2022, for 12- to 17-year-olds show:
- 32,386 adverse events, including 1,834 rated as serious and 44 reported deaths.
- 62 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 97% of cases attributed to Pfizer’s vaccine.
- 655 reports of myocarditis and pericarditis with 643 cases attributed to Pfizer’s vaccine.
- 166 reports of blood clotting disorders with all cases attributed to Pfizer. VAERS reported 167 cases of blood clotting disorders in the 12- to 17-year-old age group last week.
- 20 cases of postural orthostatic tachycardia syndrome (POTS) with all cases attributed to Pfizer’s vaccine.
U.S. VAERS data from Dec. 14, 2020, to June 17, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 53% of those who died were male, 42% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of June 17, 5,592 pregnant women reported adverse events related to COVID-19 vaccines, including 1,748 reports of miscarriage or premature birth.
- Of the 3,614 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 892 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 27% to J&J.
- 2,290 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,726 reports of myocardial infarction.
- 14,118 reports of blood-clotting disorders in the U.S. Of those, 6,313 reports were attributed to Pfizer, 5,065 reports to Moderna and 2,703 reports to J&J.
- 4,060 cases of myocarditis and pericarditis with 2,596 cases attributed to Pfizer, 1,441 cases to Moderna and 172 cases to J&J.
- 11 cases of Creutzfeldt-Jakob disease with 5 cases attributed Pfizer, 5 cases to Moderna and 1 case to J&J.
- 267 cases of POTS with 165 cases attributed to Pfizer, 84 cases to Moderna and 17 cases to J&J.
CDC advisors recommend Moderna shot for children ages 6 through 17
The CDC’s vaccine advisory panel unanimously voted 15 to 0 to recommend two doses of Moderna’s COVID-19 vaccine for children ages 6 through 17 years old.
Members of the panel acknowledged there is a risk of heart inflammation associated with both mRNA COVID-19 vaccines, but they said a follow-up survey suggests most fully recover.
Not everyone agrees, including University of British Columbia professor Dr. Steven Pelech, who last year criticized health agencies’ relaxed attitude about myocarditis as misleading.
“Contrary to what a number of people have said, there is no such thing as ‘mild myocarditis,’” Pelech said.
Pelech explained that once the heart muscle cells are killed, “they can never be replaced by new muscle cells, but only by scar tissue.” This can lead to “a greater chance of heart attack and other problems later in life.”
The FDA last week authorized Moderna’s COVID-19 vaccine for emergency use in the child and adolescent age group.
Dr. Tom Shimabukuro, deputy director of the H1N1 Vaccine Task Force at the CDC, said the risk of myocarditis “may be higher” with the Moderna vaccine compared to Pfizer, but there are limitations to what scientists know about the condition in this age group.
Shimabukuro said most adverse events reported following vaccination are “mild and transient events like injection site or systemic reactions,” and the CDC would continue to monitor the safety of COVID-9 vaccines.
CDC admits it never monitored VAERS for COVID vaccine safety signals
In response to a Freedom of Information Act (FOIA) request submitted by Children’s Health Defense (CHD), the CDC last week admitted it never analyzed VAERS for safety signals for COVID-19 vaccines.
The CDC is supposed to mine VAERS data for safety signals by calculating what are known as proportional reporting ratios (PRRs).
This is a method of comparing the proportion of different types of adverse events reported for a new vaccine to the proportion of those events reported for an older, established vaccine.
If the new vaccine shows a significantly higher reporting rate of a particular adverse event relative to the old one, it counts as a safety signal that should then trigger a more thorough investigation.
According to a briefing document, the CDC “will perform PRR data mining on a weekly basis or as needed.”
Yet in its response to CHD’s FOIA request, the agency wrote, “no PRRs were conducted by CDC” and data mining is “outside of the agency’s purview.” The agency suggested contacting the FDA, which was supposed to perform a different type of data mining, according to the briefing document.
Reports of chickenpox, shingles following COVID-19 vaccines on the rise
Doctors and scientists are seeing an increase in the reactivation of the varicella-zoster virus, which causes chickenpox, following COVID-19 vaccines, The Epoch Times reported.
After a person gets chickenpox, the virus lies dormant in the nervous system for life and can be reactivated, showing up as shingles, or herpes zoster, later in life.
Federal health officials said there’s no correlation between COVID-19 vaccines and shingles, but numerous studies show a higher incidence of shingles in people who received the vaccine.
The FDA claims it has not detected any safety signals regarding shingles following approved or authorized COVID-19 vaccines. The CDC alleges “there is no current connection” between COVID-19 vaccines and the reactivation of the chickenpox virus.
Scott Pauley, CDC spokesperson, said any adverse reactions experienced after receiving the shot are “temporary and a positive sign that the vaccine is working.”
Pfizer, Moderna COVID vaccines may increase risk of infection
A new peer-reviewed study shows two doses of an mRNA COVID-19 vaccine yield negative protection against symptomatic SARS-CoV-2 infection, while previous infection without vaccination offers about 50% immunity.
The findings, published June 15 in the New England Journal of Medicine, analyzed information from more than 100,000 Omicron-infected and non-infected residents in Qatar from Dec. 23, 2021, through Feb. 21, 2022.
Researchers found those who had a prior infection but had not been vaccinated had 46.1% and 50% immunity against the BA.1 and BA.2 Omicron subvariants more than 300 days after the previous infection.
However, individuals who received two doses of the Pfizer and Moderna vaccines, but were not previously infected, had negative immunity against the subvariants — indicating an increased risk of infection compared to someone without prior infection and vaccination.
Six months after the second dose of Pfizer, immunity against any Omicron infection dropped to -3.4% below an average person without infection and vaccination, which as a control, was set at 0.
For two doses of Moderna, immunity against any Omicron infection dropped to -10.3% about six months after the last dose.
Pfizer COVD-19 vaccine reduces sperm count, study shows
A peer-reviewed study published June 17 in the journal Andrology shows Pfizer’s COVID-19 vaccine reduced sperm concentration after the second dose.
In a retrospective longitudinal multicenter comparison study, researchers analyzed 220 semen samples of 37 donors from sperm banks in Israel.
The study participants received two doses of Pfizer’s COVID-19 vaccine, were negative for SARS-CoV-2 and did not have COVID-19 symptoms.
The changes in sperm concentration, semen volume, sperm motility and total motility count after the second dose were assessed at various study phases.
The authors concluded the negative effect of the Pfizer vaccine on sperm quality was temporary. Yet, the actual data calculating the average of values showed sperm counts had not returned to normal after five months, the end of the monitoring period.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.

