13-Year-Old Michigan Boy Dies 3 Days After Second Dose of Pfizer Vaccine, Aunt Says ‘Moral, Ethical, Health’ Questions Need Answers
Initial autopsy results showed the previously healthy boy suffered from myocarditis, an inflammatory heart condition CDC officials have acknowledged is "likely" linked to mRNA COVID vaccines.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
A 13-year-old Michigan boy died June 16, three days after he received his second dose of Pfizer’s COVID vaccine.
Preliminary autopsy results indicated that following his vaccination, Jacob Clynick’s heart became enlarged and was surrounded by fluid — symptoms similar to those documented in other teen boys who experienced myocarditis following COVID vaccination.
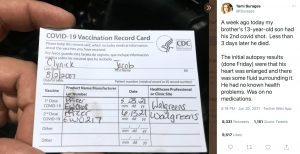
On June 20, Clynick’s aunt, Tami Burages, posted a tweet with a photo of her nephew’s vaccination card and this statement, though she has since removed this tweet:
“A week ago today my brother’s 13-year-old son had his 2nd covid shot. Less than 3 days later he died. The initial autopsy results (done Friday) were that his heart was enlarged and there was some fluid surrounding it. He had no known health problems. Was on no medications.”
Burages also tweeted, which she also removed:
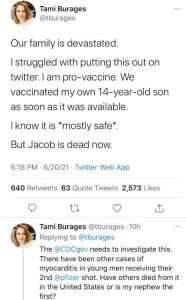
“Our family is devastated. I struggled with putting this out on Twitter. I am pro-vaccine. We vaccinated my own 14-year-old son as soon as it was available. I know it is *mostly safe*.
“The @CDCgov needs to investigate this. There have been other cases of myocarditis in young men receiving their 2nd @pfizer shot. Have others died from it in the United States or is my nephew the first?
“Should any innocent child be a sacrificial lamb in this endeavor? There are moral, ethical and health questions that need to be answered. If Jacob had not received the 2nd shot, we believe he would be alive today.”
The teen’s death was reported to the Centers for Disease Control and Prevention (CDC) and is under investigation by federal health regulators to determine if there is a correlation between the death and vaccination — according to the Saginaw County Health Department — which received notice from the Saginaw Medical Examiner’s office of the boy’s death.
“Loss of life in an adolescent for any reason is heartbreaking,” the health department said in a statement. “Health officer Chris Harrington, MPH, and medical director Delicia Pruitt, MD, are mothers of children near the boy’s age, so it hits close to home for them.”
As of Wednesday, the CDC’s Vaccine Adverse Event Reporting System (VAERS) did not include an entry for a Michigan teen who died in June. According to various reports, there is a two-month data lag between reports made to VAERS and those published to the public.
FDA to add warning about heart inflammation to Pfizer and Moderna vaccines
During a June 23 meeting, the CDC’s Advisory Committee on Immunization Practices (ACIP) said there is a “likely association” of “mild” heart inflammation in adolescents and young adults after vaccination.
The U.S. Food and Drug Administration said it will add a warning to COVID vaccines produced by Pfizer and Moderna about rare cases of heart inflammation in adolescents and young adults.
During the June 23 ACIP meeting, members acknowledged more than 1,200 cases of heart inflammation in 16- to 24-year-olds, mostly occurring in men. However, they said the benefits of vaccines outweigh the risks.
Dr. Tom Shimabukuro, deputy director of the CDC’s Immunization Safety Office, said in a presentation that data from one of the agency’s safety monitoring systems — Vaccine Safety Datalink (VSD) — suggests a rate of 12.6 cases per million in 12- to 39-year-olds during the three weeks after the second shot.
During the meeting’s public comment session, commenters chastised the CDC and its advisory committee for claiming the benefits of experimental COVID vaccines outweigh the risks in teens, when teens have a relative zero risk of dying from COVID, and are at a very low risk of experiencing adverse events.
Dr. Meryl Nass, an internal medicine physician, pointed out several flaws in the data used during the ACIP’s presentation:
“As of now, two major ways the rate of myocarditis were minimized [during the presentation] was to lump people from age 39 and down –– even though the highest rates [of myocarditis] are in the youngest kids. This waters down the rate. The other method was to only include a very narrow window of time after vaccinations started in the 12-15 age group, thus omitting the vast majority of second doses, which is when about 75% or more of the myocarditis cases occur. Also, the genders were sometimes mixed. And rates in girls are much lower than boys.”
During the presentation, Dr. Megan Wallance stated the overall efficacy of Pfizer’s COVID vaccine in the 12 to 15 age group is 100% and Moderna’s was comparable. Wallace then did a risk/benefit analysis comparing myocarditis cases versus hospitalization rates for COVID in people between the ages of 12 and 29.
“The problem with her analysis is that now the myocarditis rate used is too low. But the risk from COVID is magnified,” Nass said.
Other public commenters accused the CDC of withholding VAERS data and delaying publication of adverse event reports. Actual numbers, one commenter estimated, are three to 14 times higher than what’s been made public to date.
Physician Dr. Leslie Moore said current VAERS data is atrocious and frightening:
“All we have is the VAERS system, which is voluntary self-reporting. We know VAERS only captures 1-10% of all adverse events. Adverse events are grossly under-reported for a variety of reasons. I looked at open VAERS this morning. These products have amassed 6,000 deaths and 20,000 hospitalizations in the U.S, alone –– which is more than the other 70 vaccines for the last 30 years combined. That is with gross under-reporting and a two-month backlog. Let’s face it, these vaccines are not safe.”
The latest data from VAERS show 1,117 cases of myocarditis and pericarditis (heart inflammation) in all age groups reported in the U.S. following COVID vaccination between Dec.14, 2020 and June 11, 2021. Of the 1,117 cases reported, 686 cases were attributed to Pfizer, 391 cases to Moderna and 36 cases to Johnson & Johnson’s COVID vaccine.
Though Shimabukuro, during the June 23 ACIP meeting, cited VSD data about the rate of heart inflammation in 12- to 39-year-olds, The Defender has been unable to report on VSD data related to COVID vaccine adverse events, including heart inflammation. That’s because unlike VAERS, the VSD does not make data collected through the system readily available to the public.
The VSD is a collaborative project between the CDC and “several large health plans,” according to its website. Though the public can’t access the VSD data, there is a process whereby researchers can apply to access data.
According to the VSD website:
“There are several ways interested researchers can access VSD data. In 2002, the VSD established a data sharing program at the National Center for Health Statistics (NCHS) Research Data Center (RDC) to allow external Guest Analysts to (1) conduct new vaccine safety studies using VSD data files available at CDC or (2) to reanalyze study-specific datasets from published VSD studies.”
The VSD data sharing program is a three-step process:
- Submission of proposals to CDC’s RDC at NCHS
- Submission of proposals to VSD site Institutional Review Boards (IRBs)
- Use of CDC’s RDC at NCHS

