CDC Investigating 3 Deaths After J&J Vaccine, New VAERS Data Include 584 More Reports of Deaths After COVID Vaccines
VAERS data released today showed 86,080 reports of adverse events following COVID vaccines, including 3,186 deaths and 10,152 serious injuries between Dec. 14, 2020 and April 16, 2021.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
Data released today by the Centers for Disease Control and Prevention (CDC) on the number of injuries and deaths reported to the Vaccine Adverse Event Reporting System (VAERS) following COVID vaccines showed a notable increase in reports of injuries and deaths compared with last week’s numbers.
VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Every Friday, VAERS makes public all vaccine injury reports received as of a specified date, usually about a week prior to the release date. Today’s data show that between Dec. 14, 2020 and April 16, a
total of 86,080 total adverse events were reported to VAERS, including 3,186 deaths — an increase of 584 over the previous week — and 10,152 serious injuries, up 1,867 since last week.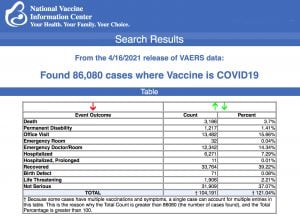
Of the 3,186 deaths reported as of April 16, 26% occurred within 48 hours of vaccination, 17% occurred within 24 hours and 41% occurred in people who became ill within 48 hours of being vaccinated.
In the U.S., 202.3 million COVID vaccine doses had been administered as of April 16. This includes 89 million doses of Moderna’s vaccine, 105 million doses of Pfizer and 8 million doses of the Johnson &Johnson (J&J) COVID vaccine.
This week’s VAERS data show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 44% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 75.9 and the youngest death reported was an 18-year-old. There are a few reported deaths in children under 18, including a 5-month old who died of a rare blood clot two days after the mother received her second dose of Pfizer vaccine and a 2-year-old, but these reports have not been confirmed.
- As of April 16, 462 pregnant women reported adverse events related to COVID vaccines, including 132 reports of miscarriage or premature birth.
- Of the 820 cases of Bell’s Palsy reported, 55% of cases were reported after Pfizer-BioNTech vaccinations, 41% following vaccination with the Moderna vaccine and 24 cases (6%) of Bell’s Palsy were reported in conjunction with J&J.
- There were 92 reports of Guillain-Barré Syndrome with 50% of cases attributed to Pfizer, 40% to Moderna and 13% to J&J.
- There were 24,841 reports of anaphylaxis with 43% of cases attributed to Pfizer’s vaccine, 47% to Moderna and 10% to J&J.
New reports of blood clots as CDC panel votes to resume J&J vaccine
Blood clotting disorders have been reported following vaccination with Pfizer, Moderna, AstraZeneca and J&J COVID vaccines. In the U.S., the J&J vaccine, marketed under the company’s Janssen subsidiary, was paused April 13 while U.S. health officials investigated reports of rare blood clots.
The CDC’s Advisory Committee on Immunization Practices (ACIP) Friday voted 10 – 4 to lift the pause and recommended continued use for persons 18 years of age and older. The panel did not recommend adding any extra warning about the risk of rare blood clotting disorders.
The ACIP said the link between blood clots and J&J’s COVID vaccine was “plausible,” but concluded the vaccine’s benefits still outweigh the risks. The recommendation by the ACIP has to be approved by the CDC and the U.S. Food and Drug Administration (FDA) before becoming official government policy, USA TODAY reported.
The Daily News reported today the CDC is investigating the deaths of two more women from a rare blood clotting disorder possibly linked to the J&J vaccine. The CDC’s advisory panel found 15 women who were diagnosed with rare blood clots, including three deaths, seven who remain hospitalized and five recovering at home, according to a slide presentation shared at today’s committee meeting.
Only two of the women were older than 50, with the risk highest in women ages 30-39, according to the CDC’s Advisory Committee on Immunization Practices. The findings appear to confirm the suspicion that younger women are more vulnerable to developing blood clots.
The Hill reported today the CDC is reviewing the death of an Oregon woman who died after receiving J&J’s vaccine. The woman, in her late 50s, was vaccinated before the state issued a pause. Two weeks after receiving the vaccine, she developed a rare but serious blood clot associated with very low platelets. The Oregon Health Authority said it was notified about the death on April 20, two days after the CDC was notified on April 18.
U.S. health authorities also are investigating the report of a Texas woman who was hospitalized after receiving J&J’s vaccine. The Department of State Health Services told The Hill in a statement that the CDC notified state officials on Wednesday afternoon of a “possible case in Texas reported through VAERS. Symptoms reported “appear to be consistent with the six cases reported elsewhere last week,” the department said.
On April 21, The Defender reported that a Nevada teen underwent three brain surgeries to repair blood clots she developed about a week after receiving J&J’s COVID vaccine. Emma Burkey, 18, suffered seizures, was placed in an induced coma and on a respirator before undergoing surgeries for a massive brain injury.
Burkey was one of the initial six cases under review by the CDC. A CDC panel said Burkey and other women experienced headaches and back pain prior to the discovery of blood clots, and also disclosed she was given heparin, a blood thinner which typically is standard treatment for blood clots, but in cases like Burkey’s, can make the condition worse.
Children’s Health Defense queried the VAERS data for a series of adverse events associated with the formation of clotting disorders and other related conditions. VAERS yielded a total of 1,123 reports for all three vaccines from Dec. 14, 2020, through April 16.
Of the 1,123 cases reported, there were 512 reports attributed to Pfizer, 448 reports to Moderna and 160 reports to J&J — far more than the six J&J cases U.S. health officials were originally investigating.
As The Defender reported April 20, the European Medicines Agency (EMA) said Tuesday it found a “possible link” between the J&J COVID vaccine and blood clots, but concluded the vaccine’s benefits outweigh the risks.
EMA’s safety committee (PRAC) said a warning should be added to the product label, but the blood clot-related disorders should be listed as “very rare” side effects of the vaccine. J&J had delayed the vaccine’s rollout in the EU after U.S. health officials paused the vaccine.
Woman paralyzed after Pfizer vaccine
The Defender reported this week that a healthy 33-year-old woman in Pennsylvania experienced paralysis 12 hours after getting her first dose of the Pfizer COVID vaccine. Doctors at the Cleveland Clinic performed a series of tests, but said they didn’t know what caused the woman to develop paralysis.
Although the woman, who asked to remain anonymous, regained feeling and strength in her arms, as of April 21, she had no function from her lower chest down besides very slight movement in a few toes. The woman’s family confirmed with Channel 11 that her case was reported to Pfizer.
CDC ignores The Defender, no response after 46 days
According to the CDC website, “the CDC follows up on any report of death to request additional information and learn more about what occurred and to determine whether the death was a result of the vaccine or unrelated.”
The Defender reached out to the CDC on March 8 with a written list of questions about reported deaths and injuries related to COVID vaccines, the status of ongoing investigations reported in the media, if autopsies are being done, the standard for determining whether an injury is causally connected to a vaccine and education initiatives to encourage and facilitate proper and accurate reporting.
After repeated attempts to obtain a response, a representative from the CDC’s Vaccine Task Force contacted us March 29 and said she had never received our list of questions — even though employees we talked to several times said their press officers were working through the questions and confirmed the representative had received them. We provided multiple deadlines, none of which were met. The Defender also provided the list again and attempted to follow-up multiple times with no response.
After repeated calls to the CDC’s media department this week, we were told the COVID response unit would be informed we had still not received answers. The person we spoke with did not know why our inquiries were being ignored. We also asked why the CDC appeared to have the ability to respond to other news media outlets in a timely manner and questioned why the agency, funded by taxpayer dollars, appeared to be selectively responding to inquiries. No answer was provided.
We were told someone would get back to us. It has been 46 days since our original email was sent inquiring into VAERS data and reports.
New study shows Pfizer’s COVID vaccine may trigger herpes virus that causes shingles
The Defender also reported this week that Pfizer’s COVID vaccine may trigger a herpes virus that causes shingles. A recent study published in the journal Rheumatology found six women out of 491 patients developed a skin rash known as herpes zoster (HZ) infection — or shingles — within three to 14 days of receiving either the first or second dose of the Pfizer’s COVID vaccine. Five of them developed shingles infection after the first dose and one after the second.
Lead researcher Dr. Victoria Furer said five of the six patients were young, had mild cases of autoimmune disease and were taking little if any medications for it — which means they should not have been at increased risk for developing the infection, as HZ tends to develop more in people over the age of 50.
“We cannot say the vaccine is the cause at this point,” Furer said. “We can say it might be a trigger in some patients.” She said further research, including a larger epidemiological study, would be needed to prove cause and effect.
Third dose of COVID vaccines and annual boosters are on the way
The Defender reported this week, vaccine makers told investors and the media that COVID booster shots are in the works. But some independent scientists warn trying to outsmart the virus with booster shots designed to address the next variant could backfire, creating an endless wave of new variants, each more virulent and transmissible than the one before.
Dr. Ozlem Tureci, co-founder and CMO of BioNTech, which developed the vaccine with Pfizer, said she also expects people will need to get vaccinated against COVID annually, like the seasonal flu. That’s because, she said, scientists expect vaccine-induced immunity against the virus will decrease over time, CNBC reported.
Tureci’s comments came after Pfizer CEO Albert Bourla said in an interview that aired April 15 that people would likely need a booster shot, or third dose, of the COVID vaccine within 12 months of getting fully vaccinated with possible additional shots each year.
J&J vaccine manufacturer Emergent BioSolutions shuts down
The Defender reported this week a J&J COVID vaccine manufacturing plant, where an ingredient mix-up last month resulted in 15 million doses of J&J vaccine being discarded, may have contaminated additional doses. A report released Wednesday by the FDA also identified a series of other problems at the Baltimore facility owned by Emergent BioSolutions.
Emergent, which in June received $628 million in taxpayer funding through the U.S. Department of Health and Human Services to establish the primary U.S. manufacturing facility for J&J’s and AstraZeneca’s COVID vaccines, agreed this week to temporarily shut down operations.
A congressional investigation was launched Tuesday into the company’s federal vaccine contract and shareholders filed a class action lawsuit Monday for false and misleading statements that drove up stock prices and misleading statements regarding the company’s readiness to mass-produce COVID vaccines.
According to CNBC, an FDA inspection of the Baltimore plant in April 2020 revealed Emergent lacked necessary personnel to produce a COVID vaccine. Another inspection, in June 2020, determined Emergent’s plan for producing vaccines was inadequate due to poorly trained staff and quality control problems, raising questions as to why the company did not fix problems earlier and why federal officials who oversaw its contracts did not demand better performance.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.

