Please go to this document for insight into the new Arexvy RSV vaccine. Where do they get these dreadful names? Go the end to get House MD segue for some laughs. Worldsaurusneezasil. Or, AREXVY.
A few pages laytare… I want to call specific attention to point 8.4 on Pediatric Use since it clearly states in this government-issued document that AREXVY would be unsafe in babies (less than 2 years old) based on what happened to the mice in the animal studies. Animal studies always precede human clinical trials as models for what might happen in the human setting. It also states that there is no safety data for young individuals ages 2-17 meaning that the consequences are unknown. On that subject matter, if you head to the clinical trial for this drug, NCT04886596, and more specifically, the history of changes made, you’ll notice 2 noteworthy things.
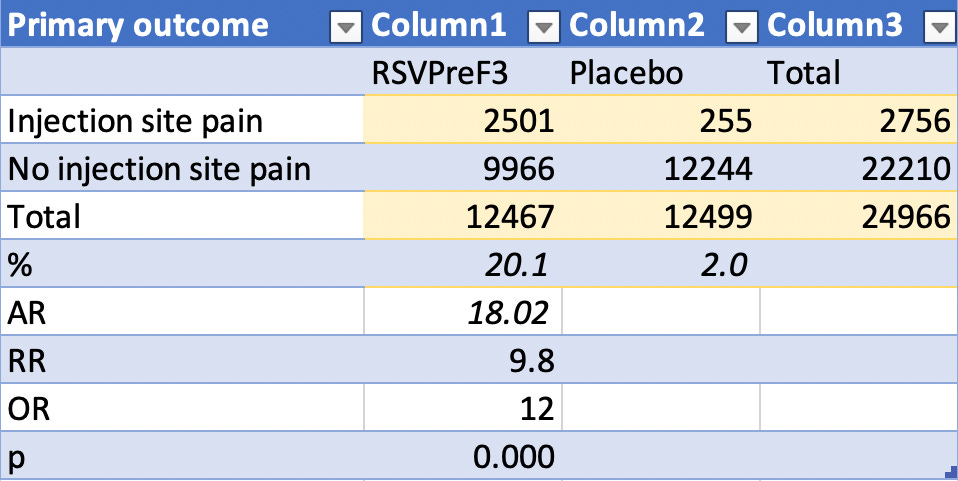
The list is long. In fact, too long to post here. I made a spreadsheet and filtered out all of the adverse events that had less than 49 reports in the drug arm (0.39%). I chose 49 since there was 49 reports of serious adverse events and wanted to make sure that ‘Death’ made the list to make a point. I made a bar graph having calculated the percentages of each as per total number of RSVPreF3 participants (12,467) versus Placebo participants (12,499). The chart is shown below. As part of my exchanges with a fellow investigator (who prompted my investigation into this subject matter), I wrote the following:
What are the excipients in the RSVPreF3 shot - not present in the Placebo - that would induce pain upon injection in 1/5 of the people injected? Is it an adjuvant? I don’t know yet. The difference is statistically significant.¹ I also find it odd that 58 individuals died in the Placebo arm. That’s 9 more than died in the drug arm. Why? These are healthy individuals. They have to be in order to qualify for participation in a Phase III clinical trials. I don’t know about my readers, but that sounds like a lot of people to have died from the effects of a placebo in a clinical trial context. It sounds like a lot of people, in general, to have died in the context of a clinical trial. This product has been approved by the FDA for use in individuals 60 years and older as of May 3, 2023. My concern here is that the age grouping will get increasingly lower for approval, until this product also gets onto the childhood vaccination schedule as the COVID-19 products did. In this FDA news release, the following is written:
In addition to this limited information on potential side effects, they also report on acute disseminated encephalomyelitis since an individual succumbed to this and died whilst concomitantly having been injected with an influenza shot and the RSV shot. Another individual succumbed to Guillain-Barré syndrome.
So it’s nice that the FDA has these requirements, even though there are hundreds of different types of associated adverse events, including (acute) myocardial infarction and atrial fibrillation, reported as part of the clinical trial data, but my question is, are these reported side effects from the RSV shots or from previous repeated COVID-19 shots? N.B. It is also more than notable that 12 infants have died in the context of an RSV product (monoclonal antibody drug) recently, reported in an article written by Amber Baker on June 19, 2023. Please click on above photo for link to article. And if you’re in the mood, a little House MD.  1 ARR: 18.02% of patients will experience Injection site pain under RSVPreF3 that they would not have under Placebo. (95% confidence interval: [17.28%, 18.77%])
|



No comments:
Post a Comment