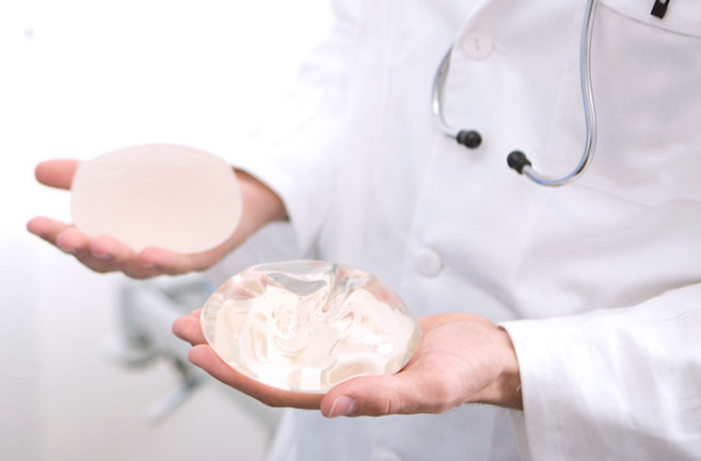
Breast implant surgery is the most common cosmetic surgical procedure in the United States, with more than 313,000 surgeries performed in 2018—a 48 percent increase since 2000.1 Silicone implants are the most common reconstructive breast surgery. In 2018, the American Society of Plastic Surgeons (ASPS) reported 101,657 breast reconstruction procedures, of which 78,814 used silicone implants.2
The U.S. Food and Drug Administration’s (FDA) Medical Device Reporting (MDR) is a post-market surveillance tool that the agency uses to monitor the performance of devices and detect any device-related safety issues, which contributes to benefit-risk assessments of medical device products.3
The FDA recently complied a report gathering data from the MDR database for all reports posted between Jan. 1, 2008 and Apr. 1, 2022 referring to adverse reactions to a saline or silicone filled breast prosthesis.4 The most common symptoms reported were fatigue (43.6 percent), joint pain (29 percent), brain fog (23.6 percent), anxiety (22.7 percent), hair loss (20.3 percent), depression (17.2 percent), autoimmune diseases (16.6 percent), rash (15.6 percent), headache (15.3 percent) and inflammation (14.7 percent).5
Breast Implant Illness Not Recognized As a Medical Diagnosis
Many patients and medical professionals use the term “breast implant illness (also known as “BII”) to describe the range of symptoms that some women report following breast implant surgery. The manifestation of BII symptoms has been reported with all types of breast implants regardless of the type of implant. Onset of reported BII symptoms ranges anywhere from immediately after surgery to years later.
Despite BII symptoms being very common among women with breast implants, breast implant illness is not recognized as a formal medical diagnosis and there are no specific tests or recognized criteria for medical professionals to make an official diagnosis.6
FDA Adds Black Box Warning to Breast Implants
In an 2019 FDA hearing, women with breast implants testified that their doctors did not warn them about the potential complications of breast implants. After the hearing, the FDA decided to restrict the sale of breast implants only to health care providers who offer patients a standardized checklist that explains risks of breast implants. The FDA now requires doctors to discuss with patients the risks involved and to give the patient an opportunity to sign off on the checklist, confirming they were informed about the risks.7
Jessica Everett, a businesswoman based in Houston, Texas who has breast implants, said:
The takeaway message is that it is truly dangerous for us getting the implants because we don’t know how they’re going to affect us. For some women, they don’t affect them—my mother has implants, and they have not affected her—but they have affected me. The medical community just needs to be educated on this in order to help other women with diagnosis and prevention.8
The FDA’s new warning states that all approved breast implants undergo safety testing prior to approval but, even so, there are risks associated with all breast implants. Some of these risks include additional surgeries, lymphoma, breast implant illness, scar tissue that squeezes the implant, breast pain, rupture of implants and infection.9
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:
No comments:
Post a Comment