664 Reports of Myocarditis in 5- to 17-Year-Olds After COVID Shots, VAERS Data Show
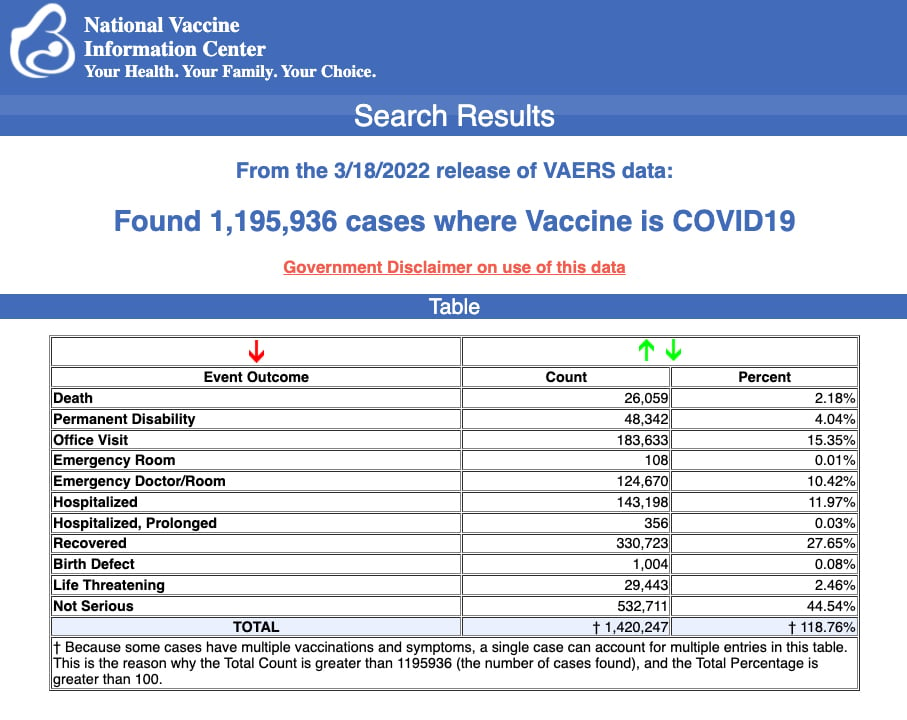
VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,195,396 reports of adverse events from all age groups following COVID vaccines, including 26,059 deaths and 211,584 serious injuries between Dec. 14, 2020, and March 18, 2022.
Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,195,396 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and March 18, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 26,059 reports of deaths — an increase of 418 over the previous week — and 211,584 reports of serious injuries, including deaths, during the same time period — up 3,375 compared with the previous week.
Excluding “foreign reports” to VAERS, 795,783 adverse events, including 11,943 deaths and 77,404 serious injuries, were reported in the U.S. between Dec. 14, 2020, and March 18, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 11,943 U.S. deaths reported as of March 18, 17% occurred within 24 hours of vaccination, 21% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 558 million COVID vaccine doses had been administered as of March 18, including 329 million doses of Pfizer, 210 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to March 18, 2022, for 5- to 11-year-olds show:
- 9,463 adverse events, including 228 rated as serious and 5 reported deaths.The most recent death involves a 7-year-old boy (VAERS I.D. 2152560) from Washington who died 13 days after receiving his first dose of Pfizer’s COVID vaccine when he went into shock and suffered cardiac arrest. He was unable to be resuscitated and died in the emergency department.
- 16 reports of myocarditis and pericarditis (heart inflammation).The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department.
- 36 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to March 18, 2022, for 12- to 17-year-olds show:
- 30,591 adverse events, including 1,755 rated as serious and 42 reported deaths.The most recent deaths involve a 17-year-old boy (VAERS I.D. 2171083) from Illinois with Duchenne muscular dystrophy who died from cardiac arrest after receiving his second dose of Pfizer’s COVID vaccine, and a 14-year-old boy from Guam (VAERS I.D. 2157944) who died one week after his first dose of Pfizer when he suddenly committed suicide.The boy’s VAERS report states:
“Sudden suicide one week after the vaccine. Patient was a perfectly happy child. After the vaccine, he became much more tired and achy and lost interest in doing his sports. One week later, without any warning, he hung himself.”
- 68 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 648 reports of myocarditis and pericarditis, with 636 cases attributed to Pfizer’s vaccine.
- 163 reports of blood clotting disorders, with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to March 18, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 72.7.
- As of March 18, 5,294 pregnant women reported adverse events related to COVID vaccines, including 1,679 reports of miscarriage or premature birth.
- Of the 3,621 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 869 reports of Guillain-Barré syndrome, with 41% of cases attributed to Pfizer, 30% to Moderna and 28% to J&J.
- 2,371 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,647 reports of myocardial infarction.
- 13,602 reports of blood-clotting disorders in the U.S. Of those, 6,077 reports were attributed to Pfizer, 4,848 reports to Moderna and 2,633 reports to J&J.
- 4,070 cases of myocarditis and pericarditis with 2,502 cases attributed to Pfizer, 1,381 cases to Moderna and 177 cases to J&J’s COVID vaccine.
Mother calls for more vaccine studies after 12-year-old experiences severe pericarditis
An Australian mother is calling for long-term studies on mRNA COVID vaccines and better advice for parents after her 12-year-old son was hospitalized for pericarditis just hours after getting the Moderna shot.
The mother, referred to in the media only as “Nat,” vaccinated her son despite being hesitant about the long-term health risks because she believed she was doing the right thing. But within seven hours of being vaccinated, her son was unable to sit or lie down without severe chest pain and complained of breathing difficulties.
ER doctors confirmed Nat’s son had pericarditis, a condition characterized by inflammation of the membrane around the heart.
Nat said she was angry because she was hesitant about giving him the vaccine, but still chose to vaccinate him anyway. Now, she wants the federal government to present better information to parents on the risks of COVID vaccines to help them make a more informed choice on whether to immunize their children.
CDC removes tens of thousands of deaths ‘accidentally’ attributed to COVID
The CDC on March 15 removed from its data tracker website tens of thousands of deaths attributed to COVID, including nearly a quarter of the deaths attributed to children. The CDC said it made adjustments to the mortality data because its website’s algorithm was “accidentally counting deaths that were not COVID-19-related.”
Prior to the adjustment on March 15, the CDC reported 851,000 COVID deaths, including 1,755 pediatric deaths. After the change, COVID-related deaths dropped to 780,000.
The change resulted in the removal of 72,277 deaths previously reported across 26 states, including 416 pediatric deaths — a reduction of 24% to 1,341, the agency said.
According to The Guardian, the error arose from two questions the CDC asks states when they report COVID fatalities. One data field asks if a person died “from illness/complications of illness,” and the field next to it asks for the date of death.
When the answer is “yes,” then the date of death has to be provided. But if a respondent included the date of death but put “no” or “unknown” in the other field, the CDC’s system assumed the answer was an error and switched the answer to “yes.” This resulted in an overcount of COVID deaths in the demographic breakdown.
The agency said once it discovered the problem, it corrected it, but it is unknown how long inaccurate COVID deaths were reported.
The CDC’s COVID statistics, used to justify which age groups should receive vaccines, were used by U.S. health agencies to support the authorization of Pfizer’s COVID vaccine for children 5 to 11 years old.
Moderna to request authorization of COVID vaccine for kids 5 to 11
Moderna on March 23 announced plans to request Emergency Use Authorization (EUA) for its pediatric COVID vaccine, citing preliminary data showing the two-dose regimen was safe for children under age 6, but may not be effective at reducing severe COVID.
The company released partial results from its two pediatric clinical trials in 6,900 children showing the mRNA shots were only about 44% effective at preventing symptomatic infection in children 6 months to 2 years old, and only 37% effective in children aged 2 to 5.
FDA guidelines for EUA products stipulate the product must show 50% efficacy.
The company said the majority of COVID cases observed were mild and no severe disease, hospitalizations or deaths were reported among any of the children who participated in the trial, making it impossible to detect the vaccine’s protective effect against the worst outcomes.
Moderna did not report details on types of side effects except for data on children who experienced fevers. The company said about 15% of children had fevers higher than 100.4 degrees, and 1 in 500 experienced a fever higher than 104 degrees.
However, data show the vaccine may not be effective at reducing severe COVID in children, who make up only a small percentage of SARS-CoV-2 infections — most of which are asymptomatic and mild.
4th COVID shot offers little protection against infection
A small study conducted by Researchers at Sheba Medical Center in Israel found efficacy of a fourth dose of Pfizer and Moderna COVID vaccines resulted in only marginal protection from SARS-CoV-2 infection.
According to the study, published in the New England Journal of Medicine, a fourth Pfizer dose showed 30% efficacy in preventing infection and Moderna’s fourth dose showed only 11%.
The study’s authors said a fourth dose provided “moderate protection against symptomatic infection” (Pfizer = 43%; Moderna = 31%), with symptomatic infection defined as a fever lasting either more or less than 48 hours. Other systemic symptoms included fatigue, myalgia, and headache.
However, these efficacy numbers fall short of the required 50% threshold required by the FDA for EUA products in the U.S.
About 25.2% of fourth dose recipients experienced moderate-to-severe local reactions and 6.5% had moderate-to-severe systemic reactions to a second booster, while the majority of all COVID cases in participants were asymptomatic or had negligible symptoms.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.