8-Year-Old Boy Dies of MIS 7 Days After Pfizer Vaccine, VAERS Report Shows
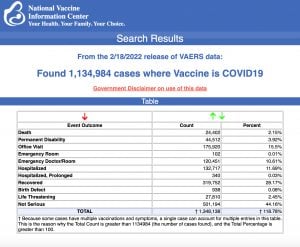
VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,134,984 reports of adverse events from all age groups following COVID vaccines, including 24,402 deaths and 196,203 serious injuries between Dec. 14, 2020, and Feb. 18, 2022.
Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,134,984 reports of adverse events following COVID vaccines were submitted between Dec. 14, 2020, and Feb. 18, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 24,402 reports of deaths — an increase of 412 over the previous week — and 196,203 reports of serious injuries, including deaths, during the same time period — up 4,286 compared with the previous week.
Excluding “foreign reports” to VAERS, 767,083 adverse events, including 11,104 deaths and 73,088 serious injuries, were reported in the U.S. between Dec. 14, 2020, and Feb. 18, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 11,104 U.S. deaths reported as of Feb. 18, 18% occurred within 24 hours of vaccination, 23% occurred within 48 hours of vaccination and 60% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 549 million COVID vaccine doses had been administered as of Feb. 18, including 323 million doses of Pfizer, 207 million doses of Moderna and 18 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed. Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to Feb. 18, 2022, for 5- to 11-year-olds show:
- 8,564 adverse events, including 188 rated as serious and 4 reported deaths.
The most recent death involves an 8-year-old boy (VAERS I.D. 2109625) from Mississippi who died 7 days after his second dose of Pfizer’s COVID vaccine when he was found blue and lifeless at home.
He was taken to the hospital with a full code in process. A pulse was detected several times, but the boy ultimately died in the ICU. It was reported to the doctor who filed the report that the boy died from multisystem inflammatory syndrome. He did not have COVID.
- 16 reports of myocarditis and pericarditis (heart inflammation).
- 30 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to Feb. 18, 2022, for 12- to 17-year-olds show:
- 29,416 adverse events, including 1,693 rated as serious and 39 reported deaths.
The most recent death involves a 13-year-old girl (VAERS I.D. 2115839) from Wisconsin who was severely compromised and received two doses of Pfizer’s COVID vaccine. Although the cause of death wasn’t clear, she appeared to have significant health issues, respiratory distress and heart problems.
- 69 reports
of anaphylaxis among 12- to 17-year-olds where the reaction was
life-threatening, required treatment or resulted in death — with 96% of
cases
attributed to Pfizer’s vaccine. - 643 reports of myocarditis and pericarditis with 631 cases attributed to Pfizer’s vaccine.
- 159 reports of blood clotting disorders, with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to Feb. 18, 2022, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 72.6.
- As of Feb. 18, 5,139 pregnant women reported adverse events related to COVID vaccines, including 1,644 reports of miscarriage or premature birth.
- Of the 3,572 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 850 reports of Guillain-Barré syndrome, with 40% of cases attributed to Pfizer, 30% to Moderna and 28% to J&J.
- 2,336 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,605 reports of myocardial infarction.
- 13,216 reports of blood clotting disorders in the U.S. Of those, 5,897 reports were attributed to Pfizer, 4,707 reports to Moderna and 2,568 reports to J&J.
- 4,021 cases of myocarditis and pericarditis with 2,475 cases attributed to Pfizer, 1,364 cases to Moderna and 171 cases to J&J’s COVID vaccine.
Autopsy confirms 24-year-old died from myocarditis caused by Pfizer vaccine
A 24-year-old college student died on Oct. 27, 2021, six weeks after receiving his second dose of Pfizer’s COVID vaccine.
George Watts, Jr., New York, needed to be vaccinated to attend college classes. He got his first Pfizer shot in August and a second dose in September.
After his second dose, Watts felt sick, started to get puffy in his face and developed a cough. He was treated at the emergency room with antibiotics for a sinus infection, but his symptoms continued to worsen.
Watts began coughing up blood, his feet and hands were hurting and he couldn’t tolerate light. Watts’ father was going to take him back to the emergency room, but his son collapsed that morning and died.
Watts’ father said his son was healthy and had no underlying health conditions.
An autopsy report from the Bradford County Coroner confirmed Watts died from “COVID-19 vaccine-related myocarditis.”
The Coroner said its office is also working on other cases in the county related to COVID vaccines and boosters.
Because Watts’ case does not meet the CDC’s case definition of myocarditis, as he did not experience “symptoms such as chest pain, shortness of breath and feelings of having a fast-beating, fluttering or pounding heart” and receive “medical tests to support the diagnosis of myocarditis and rule out other causes,” his case was not included in safety data shared by the agency with advisory panels that monitor the safety of COVID vaccines.
Family seeks compensation from government program for son’s vaccine injury
A family whose 21-year-old son developed a life-threatening reaction to Pfizer’s COVID vaccine has been waiting six months to learn if the U.S. government’s Countermeasures Injury Compensation Program (CICP) will help cover their son’s medical bills.
The CICP program, which operates under the federal Health Resources and Services Administration, provides compensation for serious injuries or death caused by certain medications, medical devices and vaccines, including COVID vaccines.
The family of Kartik Bhakta in August 2021 submitted a claim on behalf of their son. So far, the claim has been ignored.
Bhakta’s school in August stopped covering his medical expenses, which are piling up. Bhakta’s father said the ordeal has been a nightmare and nobody from the CICP has ever followed up on their claim.
The family cannot afford his future surgeries without compensation, as they had to quit their jobs to care for their son.
400,000 cases found in data analyzed by German health insurer
A German health insurer BKK ProVita (BKK) said this week an analysis of data collected from more than 10 million people suggests COVID vaccine side effects are “significantly” underreported.
The company said its analysis revealed a “significant alarm signal” and that “a risk to human life cannot be ruled out.”
Based on the data collected, BKK said the number of vaccine side effects is many times higher than the number officially announced by the Paul Ehrlich Institute (PEI), Germany’s federal health agency that monitors the safety of vaccines and biomedicines.
The PEI announced in a press release there were 244,576 suspected cases of vaccine side effects reported in 2021 following COVID vaccination, but BKK said its analysis revealed more than 400,000 cases.
BKK board member Andreas Schöfbeck told WELT, a German news publication, “The numbers determined are significant and urgently need to be checked for plausibility.”
Schöfbeck wrote an urgent letter to PEI and other agencies with a request to obtain appropriate data analyses from all health insurance companies.
CDC increases interval between COVID vaccine doses for some people
The CDC said in vaccine guidance updated Tuesday the interval between first and second doses of the Pfizer and Moderna COVID vaccines may be as long as eight weeks for some people.
The agency said an extended interval for those over age 12 may reduce the risk of myocarditis, a type of heart inflammation, in some populations.
”While absolute risk remains small, the relative risk for myocarditis is higher for males ages 12-39 years, and this risk might be reduced by extending the interval between the first and second dose,” the CDC said.
The agency continues to recommend a three- to four-week interval for the immunocompromised, adults 65 and older and others who need “rapid protection” due to an increased risk of transmission.
The CDC still recommends a second dose of Pizer three weeks after the first dose for children under 11 as it said there’s no data for that age group.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.