Nation
Nine Months Before "COVID" Broke Out, Congress Began Moving to Change Definition of "Vaccine"

If the COVID-19 outbreak was a natural thing, why did Congress begin moving to change the definition of "Vaccine" in March of 2019, a full NINE MONTHS before COVID appeared in China and (theoretically) long before anyone knew that brand new messenger RNA (mRNA) gene technology would be used for a so-called "vaccine?" Unless, of course, the outbreak was not natural and people involved in that, knew what was coming (because they were planning it), and were also planning how it would be fought; with new mRNA products?
Prior to December of 2020, a "vaccine" was considered a "Biological Product" under the US federal law. Here is how that law developed . . .
In the early 20th century, Americans were inundated with ineffective and dangerous drugs, and adulterated and deceptively packaged foods. Compounding the problem, consumers had no way of knowing what was actually in the products they bought. The passage of the 1906 Pure Food and Drugs
Act marked a monumental shift in the use of government powers to enhance consumer protection by requiring that foods and drugs bear truthful labeling statements and meet certain standards for purity and strength.While the 1906 law laid the cornerstone for the modern Food and Drug Administration (FDA), as time went on it became clear that it had major shortcomings, which limited the agency's ability to protect consumers. The law offered no way to remove inherently dangerous drugs from the market and set such a high burden of proof for misbranding, i.e., intent to defraud, that the agency was rarely able to take action against a company for fraudulent products. In addition, the law provided no authority over cosmetics, medical devices, or advertising, and imposed no standards for foods.
To help make the public aware of the 1906 law’s limitations, the FDA’s Chief Education Officer, Ruth deForest Lamb, and Chief Inspector, George Larrick, created an influential traveling exhibit in 1933 to highlight about 100 dangerous, deceptive, or worthless products that the FDA lacked authority to remove from the market. The exhibition was so shocking it was dubbed the "American Chamber of Horrors" by a reporter who accompanied First Lady Eleanor Roosevelt to view the exhibit. The name stuck. Lamb also adapted the exhibit into a 1936 book in which she explained that "All of these tragedies … have happened, not because Government officials are incompetent or callous, but because they have no real power to prevent them."
The Federal Food, Drug, and Cosmetic Act
This lead to President Franklin Delano Roosevelt’s (FDR) signing of the Federal Food, Drug, and Cosmetic Act (FDCA), 21 U.S.C. 301 et seq. which closed many of the legal loopholes highlighted in the American Chamber of Horrors and forever altered the landscape of consumer protection in America. FDR signed the Food, Drug, and Cosmetic Act on 25 June 1938.
The new law brought cosmetics and medical devices under control, and it required that drugs be labeled with adequate directions for safe use. For the first time, the FDA had authority to regulate medical devices and cosmetics, and to establish standards for foods. Drugs and devices were required to provide adequate directions for use; falsely labeled uses were misbranded; and there was no longer a need to establish intent to defraud to prove misbranding.
In addition, it became illegal to market drugs or devices that inherently endangered health, and all new drugs had to be proven safe for their labeled use before they could be marketed. Today, this important law still looms large in guiding the FDA’s mission.
One of the items the law regulated were vaccines.
Of course, the law underwent changes over the 80+ years it has been in effect. Under Section 351(i)(1) of the Public Health Service Act (42 U.S.C. 262(i)(1)) the law made clear:
The term “biological product” means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, protein (except any chemically synthesized polypeptide), or analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound), applicable to the prevention, treatment, or cure of a disease or condition of human beings. (emphasis added)
I reiterate that I added the bold-face to the phrase "protein (except any chemically synthesized polypeptide)"
What this meant is simple: all the items outlined in that paragraph were "biological products" subject to Food and Drug Administration regulation . . . except any chemically synthesized polypeptide.
Chemically synthesized polypeptides were specifically NOT "biological products."
This is important because as we fast-forward to the present day, and look at the COVID-19 situation, and specifically the new messenger RNA "vaccines" they are, in sum and in substance "Chemically synthesized polypeptides."
Under the old law, they would not be considered "biological products" and so it follows they could NOT be "vaccines" and would not / could not be regulated by the FDA.
How many Americans know this? How many Americans know that under our U.S. law, the mRNA "vaccines" did not -- and could not -- qualify as "vaccines" under what was our law for some 80+ years?
Enter United States Congressman Bill Pascrell, Democrat, from New Jersey.
What you are about to read is CRUCIAL because it shows a timeline long in advance of COVID-19 ever appearing in the world, and long in advance of any mRNA "vaccines" for COVID-19. It seems to show - seems to PROVE -- the outbreak itself and the "vaccines" were all PLANNED long in advance. It may also give rise to questions about whether or not our own government officials were involved in the planning.
In March, 2019, Congressman Pascrell Sponsored an Appropriations Bill known as:
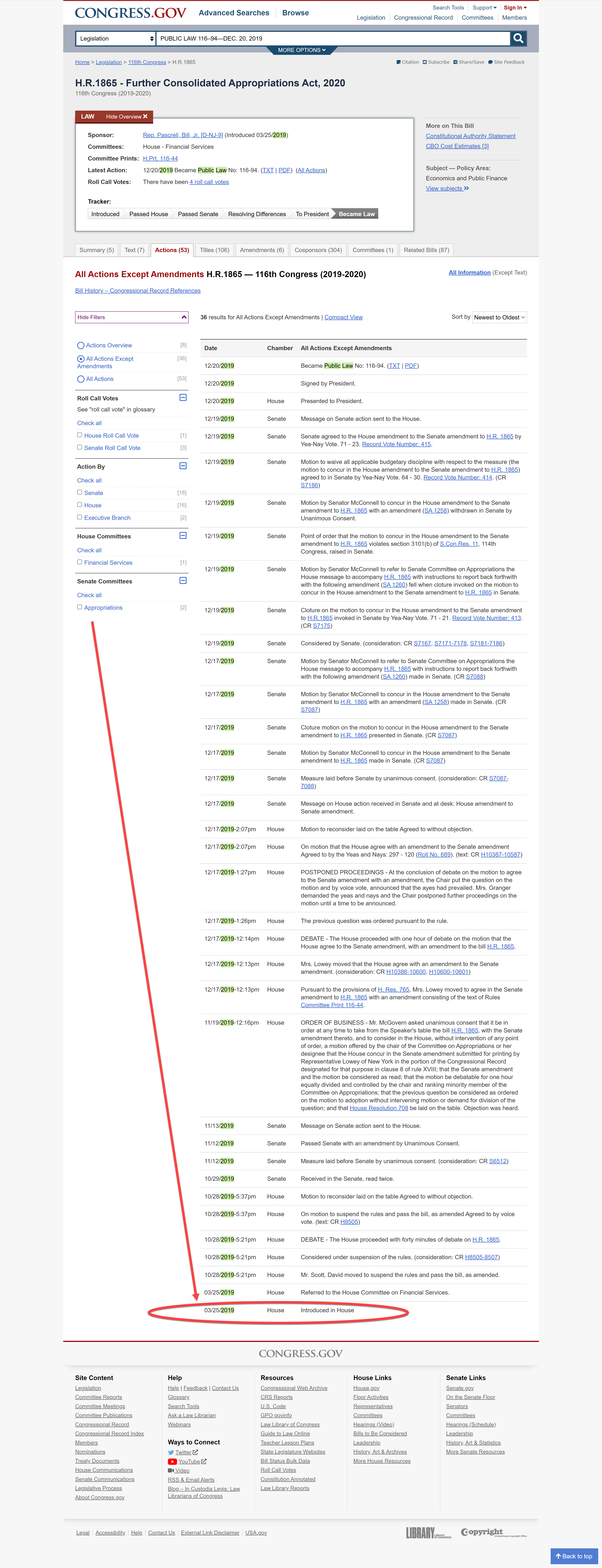
H.R.1865 - Further Consolidated Appropriations Act, 2020 (Link to Congress.gov HERE)
According to the Bill Index on the US Government web site "Congress.gov" the Bill was Introduced on March 25, 2019, but was not fully passed and signed into law until December, 2019.
Here is the Bill Index:
A direct link to the info above on the Congress.gov web site is HERE.
It is interesting to note that this Bill, is an Appropriations Bill. It is designed to spend money.
Yet contained within the original text of the original bill, introduced in March, 2019, long before COVID ever hit the world, the Bill contains THIS section:
SEC. 605. BIOLOGICAL PRODUCT DEFINITION.
Section 351(i)(1) of the Public Health Service Act (42 U.S.C. 262(i)(1)) is amended by striking “(except any chemically synthesized polypeptide).”
The direct link to the original text (March, 2019) of the Bill on Congress.gov is HERE
HERE is a direct link to a PDF of the Bill. You can find the item shown above on Page 595 of the 700+ page Bill.
The Section 605 in the PDF of the Bill looks like this:
Strange thing to put into an Appropriations bill.
Why on earth would something like this be put into an Appropriations Bill? Why put an arcane change of definition of "Biological Products" into an Appropriations Bill?
HMMMMMMMMM.
The Bill passed, and by December 20, 2019, it had been signed by President Trump and became law.
Eleven DAYS later, on December 31, 2019, a "novel coronavirus" made its appearance in Wuhan, China and started making people there, very sick.
If you type a search question into Google, and ask "When did novel coronavirus first appear in Wuhan china?" it returns the following:
So if COVID-19 wasn't even reported anywhere in the world until December 31, 2019, how did Congress know to change the definition of a "Biological Product" (i.e. "vaccines") eleven days earlier on December 20, 2019?
In fact, since COVID-19 wasn't even in existence, and mRNA "vaccines" weren't even in production for COVID-19, how did Congressman Bill Pascrell of New Jersey know to put a change of definition, which directly cleared the way for mRNA vaccines by striking out the prohibition on "any chemically synthesized polypeptide" into his original appropriations Bill in March of 2019?
What did Congressman Pascrell know in March of 2019, that lead him to use an Appropriations Bill, to change the definition of "Biological Product" that perfectly cleared the way for mRNA genetic products to then be called "vaccines?"
Some people might start to wonder if the reason Congress changed the definition might be because Congress knew this particular outbreak was coming, and knew that mRNA products would be used for it.
Some people might also start to wonder if the only way Congress could have known the outbreak was coming is because maybe they were in on it.
Maybe, the whole COVID thing, was planned? By our own government?
A government which is now trying its best to FORCE people to take a so-called "vaccine" that wouldn't have even qualified to be CALLED a "vaccine" without the convenient little change in law provided by New Jersey Congressman, Bill Pascrell.
If you think something doesn't smell right here, you're probably not alone.



No comments:
Post a Comment