CHD to Sue FDA for ‘Recklessly Endangering’ Children if Agency Authorizes Pfizer Vaccine for Children 5 to 11 Years Old
An advisory committee to the U.S. Food and Drug Administration will meet Tuesday to consider emergency authorization of Pfizer’s COVID vaccine for young children. In a letter to the FDA, Children’s Health Defense outlines why such a move would be reckless.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
Children’s Health Defense (CHD) today said it will take legal action against the U.S. Food and Drug Administration (FDA) if the agency grants Emergency Use Authorization (EUA) for the Pfizer-BioNTech SARS-CoV-2 vaccine for children aged 5-11.
In a letter signed by Robert F. Kennedy, Jr., CHD chairman and chief legal counsel, and Dr. Meryl Nass, member of the CHD Scientific Advisory Committee, Kennedy and Nass wrote:
“CHD will seek to hold you accountable for recklessly endangering this population with a product that has little efficacy but which may put them, without warning, at risk of many adverse health consequences, including heart damage, stroke, and other thrombotic events and reproductive harms.”
The letter was addressed to Dr. Arnold Monto, chairman of the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), committee members and all FDA staff.
VRBPAC members are set to meet Tuesday to consider and likely vote on whether to grant EUA for the Pfizer vaccine for 5- to 11-year-olds.
In May, the FDA authorized Pfizer’s vaccine for 12- to 15-year-olds. Moderna and Johnson & Johnson vaccines have not yet been authorized for children under 18.
The letter outlines 12 reasons the FDA should not authorize the pediatric vaccine and provides supporting evidence to back up each argument.
Read the letter:
Dear Chairman Monto, VRBPAC Members and FDA Staff:
We write to you on behalf of Children’s Health Defense (CHD), a non-profit organization devoted to the health of people and the planet. We have actively followed your work to evaluate, authorize and approve vaccines for the American public and particularly children.
We are aware that you are likely to authorize Pfizer’s BioNTech SARS-CoV-2 vaccine for children aged 5-11 at your meeting on Oct. 26. Your authorization thus will expose over 20 million children in the U.S., and millions more around the world, to potential COVID-19 vaccination of an Emergency Use Authorization (EUA) product.
We are writing to put you on notice that should you grant EUA status to this pediatric EUA vaccine, CHD is poised to take legal action against you and other Vaccines and Related Biological Products Advisory Committee (VRBPAC) voting members as well as the FDA.
CHD will seek to hold you accountable for recklessly endangering this population with a product that has little efficacy but which may put them, without warning, at risk of many adverse health consequences, including heart damage, stroke and other thrombotic events and reproductive harms.
We briefly outline why such authorization would be reckless:
1. The risks demonstrably outweigh the benefits of COVID vaccination for young children. Deaths and hospitalizations are rare and have been inflated inaccurately.
2. Nearly half of all children have natural immunity to COVID, according to the Centers for Disease Control and Prevention (CDC). There is no ethical justification for superfluous vaccination that will put children at elevated risk of vaccine harm.
3. Some children likely will die or be permanently injured from these vaccines based on the authorization for children 12-16.
4. The clinical trials for the pediatric vaccine were too small to detect safety signals for a population in the millions.
5. There are no long-term safety data for COVID vaccination of young children, making this an experiment rather than appropriate medical prevention.
6. Unethical coercive pressure will be applied to children and their parents, as has occurred with older children and adults. To grant authorization is to abet this unethical coercion that violates the Nuremberg Code’s first principle.
7. There is no available care for children injured by COVID shots. The science and medicine have not yet developed, and most families will be unable to cover the costs of potential catastrophic injuries.
8. VRBPAC members should not participate in an exercise disguising a foregone conclusion. The president’s purchase of 65 million pediatric doses, the CDC guidance for COVID vaccine delivery, the American Academy of Pediatrics’s promotion of COVID vaccination for children all call into question whether this committee’s deliberations mean anything.
If the administration is unprepared to wait for your advice, let alone heed it, you should signify your disapproval on behalf of the country the FDA is meant to protect.
9. First, do no harm. You are physicians who owe a duty to patients and medical ethics. If you authorize these shots, given all you know, will you be upholding your oath? If not, is it possible that your acts could later be seen as reason to remove your medical licenses?
10. The liability-free nature of your deliberations may not stand the test of time. In the fullness of time, your decisions may not have the liability protection that they currently enjoy. Under the PREP Act of 2005, all actors advancing an EUA agenda for medical countermeasures enjoy liability protection, absent willful misconduct.
Nonetheless, if at a later point these shots are deemed non-therapeutic gene products that you knowingly and recklessly authorized, and which were then distributed to children as a direct result of your decision, it is possible that liability could later attach.
11. There is no COVID emergency for children of this age.
12. There are safer drugs that could be used prophylactically and therapeutically for COVID in children. There is extensive and compelling medical evidence for this assertion — and the choice to eschew use of these drugs in favor of a demonstrably dangerous vaccine is arbitrary and capricious.
We ask that you carefully consider all the information above before making any recommendation to authorize Pfizer’s vaccine in the 5 through 11 year age group at your meeting on Tuesday, Oct. 26.
Sincerely yours,
Let’s investigate the basis for claims that children aged 5 through 11 need to be vaccinated for COVID.
1. The truth is that children aged 5-11 are at extremely low risk of hospitalization, death, MIS-C or Long COVID.
a. What is the actual risk of hospitalization, death and MIS-C in aged 5 through 11-year-old children? This age group has the lowest rate of severe disease and death than all other age cohorts.
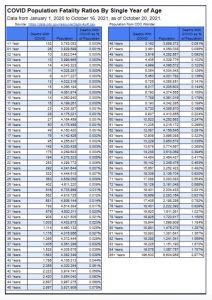
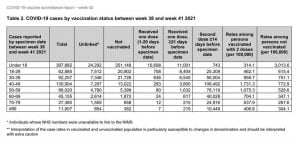
CDC reports 94 COVID-19 deaths with COVID since Jan. 1, 2020, in the 5 through 11 age group. However, CDC designates these as deaths “involving COVID” or “with COVID” rather than due to COVID, according to CDC’s chart below.
b. In the October 2021 Pediatrics, a report by David McCormick et al. showed that of 112 pediatric deaths associated with SARS-CoV-2, 86% had comorbidities, especially obesity, neurologic and developmental conditions. The mean age of decedents was 17.
c. It is impossible to separate deaths with COVID from those due to COVID in the U.S. because the CDC does not distinguish them. But what we do know is that child deaths due to COVID in Germany, according to the BILD newspaper, were 20 in May 2021, in a country with 85 million people.
Pediatric deaths were “under 30” through March 2021, according to the UK government, with 60 million people.

d. Regarding MIS-C, the data are sparse. The UK’s Joint Committee on Vaccination and Immunization stated on Sept. 3, based on data from the UK, Canada and the U.S.:
“There are no clinical trial data of vaccine efficacy against PIMS-TS [MIS-C], nor any real-world estimates of vaccine effectiveness. Post-COVID-19 syndrome (often called ‘long COVID’) has been reported in children and young people.
“Existing studies suggest that longer-term (≥8 weeks) symptoms following SARS-CoV2 infection occur in about <1% to 10% of persons after COVID-19, with controlled studies generally reporting rates at the lower end of this range.”
e. Regarding hospitalizations, we have some case series from pediatric hospitals in the U.S. In one report in Hospital Pediatrics, of 146 hospitalized pediatric COVID cases during 5 months in 2020, only 20 (14%) were deemed “significantly symptomatic.”
Only 24 of the total were actually admitted because of COVID. Of those significantly symptomatic, 60% were obese and 35% had asthma. COVID-19 was either incidental or minimally related to the reason for hospitalization in 86% of the admissions.
Of the 4 pediatric deaths in this series, only one was attributed to COVID by the authors, in a “medically complex patient admitted for respiratory failure.”
A recent CDC MMWR publication on pediatric hospitalizations contained the following graph, based on which CDC claimed massive increases in pediatric hospitalizations this summer.

However, it should be compared to another CDC graph below, updated through Oct. 16: CDC’s graph of COVID-associated hospitalization rates by age. The gray dotted line represents the 5-11 age group.
It hugs the X axis, and has already dropped back to baseline since its slight uptick in the summer. The aqua line represents the 18-49 year group, the navy line represents the 50-64 year group and the red line 65 years and above.
These graphs and detailed reports from pediatric hospitals, including the report by Lauren Kushner et al., “‘For COVID’ or ‘With COVID’: Classification of SARS-CoV-2 Hospitalizations in Children” make clear that hospitalization rates due to COVID are essentially negligible in this age group, even during peaks of infection.
The reason CDC could claim steep increases in pediatric hospitalizations was because even a handful of additional hospitalizations caused a marked increase in the rate, and because it included hospitalizations in which COVID was an incidental finding.

f. Regarding MIS-C, this is a problem whose dimensions are uncertain, and for which the role of vaccinations is unknown.
2. Pediatric vaccinations cannot be justified as necessary for herd immunity, when herd immunity itself is impossible to achieve with current vaccines. Vaccinating children to protect adults is also unethical.
a. Given the rapid waning of protection and the inability of current vaccines to prevent transmission of SARS-CoV-2, admitted by CDC Director Walensky, it is not possible to achieve herd immunity with vaccination.
In fact, the UK’s head of the Oxford Vaccine Group, Professor Sir Andrew Pollard, told Parliament that herd immunity due to vaccination was a myth, and “not a possibility.”
b. While protecting the elderly has sometimes been used as the justification for vaccinating children (for example, against influenza) it is unethical to have one group take on risk to protect another group. It is even more problematic when the group being asked to assume the risk, children, cannot give informed consent.
When the magnitude of the risk is significant (of myocarditis, for example) but has not been quantified, and the long-term risks of vaccination are unknown, demanding children shoulder this risk for others is ethically untenable.
3. We know nothing about the long-term risk of vaccination in children. The myocarditis risk immediately after vaccination in older children is considerable, potentially life-threatening, and increased exponentially with decreasing age.
a. The risk might be very high. The pediatric clinical trials are too small to quantify the risk from myocarditis and quantify the risk of most other adverse events.
FDA acknowledged, in its approval letter to BioNTech, c/o Pfizer on Aug. 23, that it was unable to assess the “known serious risks of myocarditis.”
“We have determined that an analysis of spontaneous post-marketing adverse events reported under section 505(k)(1) of the FDCA will not be sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis.
“Furthermore, the pharmacovigilance system that FDA is required to maintain under section 505(k)(3) of the FDCA is not sufficient to assess these serious risks.
“Therefore, based on appropriate scientific data, we have determined that you are required to conduct the following studies …”
FDA told BioNTech-Pfizer that since FDA was unable to assess the myocarditis risk, it expected BioNTech-Pfizer to do so. FDA wants Pfizer’s final reports on myocarditis to be submitted in 2024 and 2025.
Do you think it is okay to vaccinate tens or hundreds of millions of the world’s children before BioNTech-Pfizer tells us to what extent their vaccines damage childrens’ hearts?
b. According to the Jerusalem Post on Oct. 7, the health ministry was considering whether “individuals vaccinated with the Pfizer coronavirus vaccine may be asked to avoid strenuous exercise [including swimming] and other physical activity for one week after receiving each dose due to cases of myocarditis…”
c. Let’s review some of the data that has been presented at VRBPAC and Advisory Committee on Immunization Practices (ACIP) meetings this year.
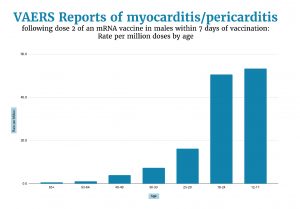
Confining ourselves to males who received a second dose of an mRNA vaccine and reported myocarditis within a week of the shot to Vaccine Adverse Event Reporting System (VAERS), we see that the reported myocarditis rate in the age group 12-17 (62.7/million) was over 100 times greater than in the over-65 group (0.6/million).
Graphing these rates (below) we see that the rates increased exponentially as age decreased. Extrapolating from this graph we could expect the highest myocarditis rates in younger children.
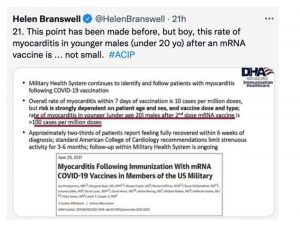
Tweeting from the Oct. 21 ACIP meeting, esteemed reporter for STAT, Helen Branswell, informed us that the rate of myocarditis in military servicemembers under age 20 was over 100 cases per million.
The third graphic is the report to VAERS of a death in a 15-year-old, attributed to myocarditis, six days after receiving a COVID vaccination.

FDA’s Doran Fink pointed out at a recent meeting that we have no idea of the rate of subclinical myocarditis. Nor are there good data regarding the long-term sequelae of COVID vaccine-induced myocarditis in teens or other children.
Recall that a 2015 military study by Renata Engler et al. revealed a rate of subclinical myocarditis after a 1st smallpox vaccine to be one in thirty.
4. The evidence suggests that Pfizer is neither reliable nor trustworthy.
a. Pfizer is projected to earn $33 billion dollars this year in vaccine sales, and more than that next year. Do you honestly think Pfizer-BioNTech will try to identify the actual rate of myocarditis in children, when so much money is at stake?
I am sure you are aware that Pfizer is the world’s largest drug company, and also that Pfizer has paid more in fines to federal and state governments than any other pharmaceutical company.
b. An Oct. 19 Public Citizen report titled Pfizer’s Power, discussing Pfizer and its COVID vaccine contracts notes:
“neither Pfizer nor the U.S. government can make ‘any public announcement concerning the existence, subject matter or terms of this Agreement, the transactions contemplated by it, or the relationship between the Pfizer and the Government hereunder, without the prior written consent of the other.’ The contract contains some exceptions for disclosures required by law.”
I recommend you read the entire brief report to understand the nature of the vaccine company whose product you are dealing with.
5. What we don’t know yet, or haven’t been told, is critically important. Why rush the shots into children?
a. Four Nordic countries recently halted the use of Moderna’s vaccine in some age groups due to the risk of myocarditis. It was reported by the Wall Street Journal that FDA paused its review of the Moderna vaccine for teenagers in response to the Nordic countries’ action.
The article was subtitled, “Agency holds off decision on expanding use of shot to 12-to-17-year-olds while it looks into risk of rare heart condition.”
Shouldn’t FDA hold off its expansion of the Pfizer shot to 5-11 year olds, since Pfizer’s shot also causes myocarditis, until it has completed this review?
b. The VAERS data on myocarditis are clearly concerning. The absence of data from other FDA and CDC accessible databases ought to be alarming.
When 60% of the U.S. has been vaccinated, how can it be that we still do not know the actual rates of myocarditis in the population? Is this information being concealed in order to garner authorizations for the vaccines in the pediatric population?
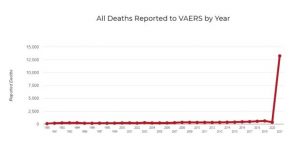
c. Other information from VAERS ought to have raised an alarm long ago. How can it be that adverse event reports input to VAERS are greater, since the COVID vaccines were rolled out, than all cumulative adverse event reports to VAERS for the prior thirty years?
Death reports for 2021 are also greater than cumulative deaths reported to VAERS over the preceding 30 years. Why has no public health official explained this?
Why has CDC, which is charged with investigating every reported death in VAERS, simply waved its hands and claimed none are due to vaccination, without providing any data?
d. The bottom line is that we have no idea of either the short or long-term risk of the Pfizer vaccine in 5- to 11-year-old children, but it is reasonable to assume the risk of myocarditis could be considerable.
Other risks have not been quantified but could also be considerable. Yet we do not even know their magnitude in adults, after 6.8 billion COVID vaccinations have been administered throughout the world.
How can anyone possibly justify vaccinating children with vaccines for which the world’s public health professionals have failed to collect and analyze the most rudimentary data on safety during the largest rollout of (mostly experimental) vaccines in the history of the world?
Obviously, policies were put in place such that we will never know the risks of COVID vaccinations nor the magnitude of those risks. Why?
6. The benefit exceeds the risk.
a. In the past, your briefers have exaggerated the harms to children from COVID and magnified the benefits of vaccination in order to claim that benefit exceeds risk.
This was accomplished through the use of datasets that inexplicably failed to yield adverse event signals, conflating deaths and hospitalizations “with” COVID as if all were “due to” COVID, ignoring the existence of naturally acquired immunity and making overly optimistic assumptions about the efficacy and duration of vaccine-induced protection.
However, if you use more realistic data, such as presented here, the risk exceeds benefit in the 12-15 age group, and will exceed benefit in the 5-11 year age group also.
b, One of your briefers who failed to find adverse event signals in the Vaccine Safety Datalink was Nicola Klein, who is the principal investigator in multiple COVID vaccine studies for Pfizer conducted in both adults and children. Those trials have brought in many millions of dollars to her institution. This conflict of interest was undisclosed.
c. What percentage of young children are already immune? At a June VRBPAC meeting, we learned that 27% of children were naturally immune, a higher proportion than in any other age group. They were tested using anti-nucleocapsid antibodies.
Since then, they have had a summer in which to play together and two months of in-person schooling, and their immunity could be approaching 50%. Vaccinating these children will expose them to risk without the prospect of benefit. Why do it?
d. FDA allows Pfizer to use anti-nucleocapsid antibody tests to identify prospective subjects for clinical trials who have preexisting immunity — they are not included in the efficacy analysis.
Yet FDA and CDC do not allow ordinary American children or adults to use the identical test to demonstrate that they are already immune and don’t need vaccinations for COVID.
Why do you think this is? Does it make medical or scientific sense? Common sense? Why are Americans forbidden from demonstrating they are immune and can safely go on with their life unvaccinated?
e. Didn’t you find it surprising that so many federal public health officials claimed that recovered immunity was expected to be weaker than vaccine-induced immunity? Didn’t that go against what you learned in medical school?
Can everyone finally agree that recovered immunity is broader and longer-lasting than immunity derived from current COVID vaccines?
7. Beware the data tricks being employed to minimize safety concerns.
a. You know that VAERS is a passive reporting system that is intended to provide signals of potential safety problems, which must be evaluated with additional studies.
You know that VAERS data cannot be used to calculate the rates of any adverse reaction. Yet CDC did exactly that for anaphylaxis, claiming the rate of VAERS reporting was the rate of occurrence.
b. When a high-quality study of Massachusetts General Hospital and Brigham hospital employees showed that anaphylaxis occurred in 250 per million employees, CDC failed to update its website and still claims, as of Oct. 18, that anaphylaxis occurs only 2-5 times per million COVID vaccinees. Which begs the question: how accurate are CDC’s other adverse event rates?
c. CDC has made a number of changes to its standard practices since the beginning of the pandemic. Here is just one example. Beginning on May 1, for CDC to accept a report of a breakthrough case, the infected case must have required hospitalization or died and had their infection confirmed with a PCR test using 28 or fewer cycles.
Other problems with data acquisition of breakthrough cases have further contributed to keeping the official number of such cases much lower than they really are. In the UK, in all age cohorts of 30 years and up, there is a higher rate of COVID cases in the vaccinated compared to the unvaccinated.
d. Maddie de Garay was a healthy 12 year old when she entered Pfizer’s pediatric COVID vaccine trial at the University of Cincinnati with her two siblings.
She became ill immediately after the second dose with high fever and then a wide range of symptoms. Over the subsequent six months she had about a dozen ER visits and six hospitalizations. She has required a feeding tube to be nourished and uses a wheelchair. Dr. Frenck, the principal investigator, was her physician and is aware of these problems.
Yet she was not reported as a serious adverse event in the trial documents, and when her trial was published in The New England Journal of Medicine (NEJM), there were no serious vaccine-related adverse events listed for any subject.
Her physician, Frenck, was the first author of the NEJM study. How many other subjects in Pfizer’s trials were similarly injured, but went unreported? How many principal investigators issued positive reports despite knowing of injuries?
8. How many vaccines will children need? What are the cumulative risks? How can we be sure there will be no antibody-dependent enhancement?
a. None of this is known. Under what conditions is it acceptable to experiment on millions of our children simultaneously?
b. In Israel, the green pass expired after 6 months, and college students have already received a booster dose. As you know, data regarding booster dose efficacy and safety is scanty. Evidence of cumulative dose safety does not exist.
9. Conflicts of interest.
a. We at Children’s Health Defense have not devoted ourselves to investigating potential VRBPAC member conflicts of interest (COI), but we noted that half the voting VRBPAC members are temporary members, presumably installed to replace permanent members who disclosed COIs.
Yet 3 current voting members have glaring COIs. Eric Rubin, editor-in-chief of the NEJM, has published all the Pfizer clinical trials, and the NEJM will have earned a considerable sum for reprints and advertising from Pfizer.
Drs. Amanda Cohn and Melinda Wharton are both career CDC employees. Were either of them to vote against a vaccine authorization or approval it could have severe consequences for their careers.
Both Rubin and Wharton are temporary members. We are dismayed that conflicted temporary members were selected to replace conflicted permanent members on the VRBPAC.
10. Early treatment works, but to acknowledge this would prevent EUAs from being issued for COVID vaccines and on-patent drugs like Regeneron’s monoclonal antibodies, remdesivir and molnupiravir.
a. The statute under which Emergency Use Authorizations (EUA) are defined requires that there exist no alternative approved, adequate and available product in order for an EUA to be issued. Had effective drugs not been deliberately suppressed, no EUAs could have been issued.
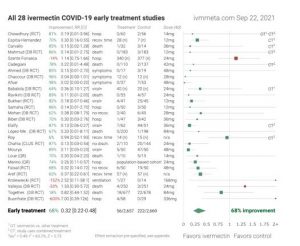
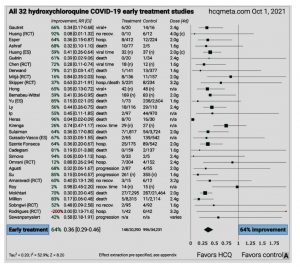
b. If children and adults were treated early with proven drug combinations, very few would progress to the inflammatory and thrombotic stage of COVID-19. While this statement may appear controversial, forest plots of the compiled literature on hydroxychloroquine and ivermectin for COVID are very compelling, with average efficacy against the different endpoints of 64% to over 80%.
c. The Nebraska Attorney General on Oct. 15 issued an extraordinary 48-page opinion regarding these two drugs, giving safe harbor to medical practitioners and pharmacists who prescribe and dispense them with informed consent.
We expect to see more opinions like it as the states protect the public from the harmful acts of health professionals.
d. The probable efficacy of chloroquine drugs for coronaviruses was demonstrated in experiments published by the CDC in 2005 and by Dr. Fauci‘s National Institute of Allergy and Infectious Diseases in 2014.
This prior knowledge, obtained by CDC and National Institutes of Health regarding these drugs’ efficacy at standard doses and their safety at standard doses, while agency officials suppressed their use during the pandemic, is clear evidence of willful misconduct and nullifies liability protection for these federal officials.
11. Spike protein, the putative antigen induced by all 3 COVID vaccines, is a toxin.
It is produced and enters the circulation, has predictable negative consequences to vascular endothelium, activates platelets and crosses the blood-brain barrier. It would be expected to trigger the destruction of cells that produce it and present it on their surfaces.
Products that induce the production of spike protein should only be used after careful consideration of the individual recipient’s risks and benefits.
They should not be employed in mass vaccination programs where there is no learned practitioner to weigh appropriate use, nor in individuals with a very low risk of serious COVID disease.

