Nearly 15,000 Deaths, More Than 700,000 Injuries Reported to VAERS Since December 2020 Rollout of COVID Vaccines in U.S.
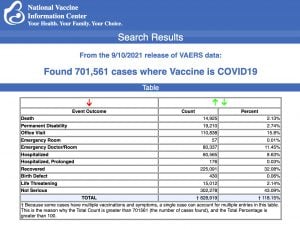
VAERS data released Sept. 17 by the CDC showed a total of 701,561 reports of adverse events from all age groups following COVID vaccines, including 14,925 deaths and 91,523 serious injuries between Dec. 14, 2020 and Sept. 10, 2021.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
Data released Sept. 17 by the Centers for Disease Control and Prevention (CDC) showed that between Dec. 14, 2020 and Sept. 10, 2021, a total of 701,561 adverse events following COVID vaccines were reported to the Vaccine Adverse Event Reporting System (VAERS). The data included a total of 14,925 reports of deaths — an increase of 419 over the previous week.
There were 91,523 reports of serious injuries, including the reports of deaths, during the same time period — up 3,352 compared with the previous week.
Excluding “foreign reports” filed in VAERS, 559,462 adverse events, including 6,756 deaths and 43,073 serious injuries, were reported in the U.S. between Dec. 14, 2020 and Sept. 10, 2021.
Of the 6,756 U.S. deaths reported as of Sept. 10, 12% occurred within 24 hours of vaccination, 17% occurred within 48 hours of vaccination and 31% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 378.2 million COVID vaccine doses had been administered as of Sept. 10. This includes: 216 million doses of Pfizer, 148 million doses of Moderna and 15 million doses of Johnson & Johnson (J&J).
The data come directly from reports submitted to VAERS, the primary government-funded system for reporting adverse vaccine reactions in the U.S.
Every Friday, VAERS makes public all vaccine injury reports received as of a specified date, usually about a week prior to the release date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
This week’s U.S. data for 12- to 17-year-olds show:
- 19,827 total adverse events, including 1,169 rated as serious and 19 reported deaths. Two of the 19 deaths were suicides.
The most recent deaths involve one report of two patients [VAERS I.D. 1655100] who died after their second dose of Pfizer, including a 13-year-old female.
Other recent reported deaths include a 15-year-old boy (VAERS I.D. 1498080) who previously had COVID, was diagnosed with cardiomyopathy in May 2021 and died four days after receiving his second dose of Pfizer’s vaccine on June 18, when he collapsed on the soccer field and went into ventricular tachycardia; and a 13-year-old girl (VAERS I.D. 1505250) who died after suffering a heart condition after receiving her first dose of Pfizer.
- 2,972 reports of anaphylaxis among 12- to 17-year-olds with 99% of cases
attributed to Pfizer’s vaccine. - 488 reports of myocarditis and pericarditis (heart inflammation) with 481 cases attributed to Pfizer’s vaccine.
- 106 reports of blood clotting disorders, with all cases attributed to Pfizer.
This week’s U.S. VAERS data, from Dec. 14, 2020 to Sept. 10, 2021, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 42% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 72.9.
- As of Sept. 10, 3,650 pregnant women reported adverse events related to COVID vaccines, including 1076 reports of miscarriage or premature birth.
- Of the 2,783 cases of Bell’s Palsy reported, 50% were attributed to Pfizer vaccinations, 42% to Moderna and 8% to J&J.
- 593 reports of Guillain-Barré syndrome, with 39% of cases attributed to Pfizer, 33% to Moderna and 27% to J&J.
- 149,681 reports of anaphylaxis with 42% of cases attributed to Pfizer’s vaccine, 51% to Moderna and 7% to J&J.
- 9,260 reports of blood clotting disorders. Of those, 3,968 reports were attributed to Pfizer, 3,376 reports to Moderna and 1,866 reports to J&J.
- 2,452 cases of myocarditis and pericarditis with 1,545 cases attributed to Pfizer, 806 cases to Moderna and 93 cases to J&J’s COVID vaccine.
FDA panel overwhelmingly rejects Pfizer boosters for healthy people 16 to 65 years old
On Sept. 17, a panel of scientific advisors to the U.S. Food and Drug Administration (FDA) voted 16 to 2 against recommending a third shot of Pfizer’s COVID vaccine for healthy people 16 and older, but voted unanimously in favor of recommending the booster shot for the immunocompromised and all people 65 or older.
The vote came after a sharp debate in which many of the panel’s independent experts, including infectious disease doctors and statisticians, challenged whether the data justified a broad rollout of extra shots when the vaccines appear to still offer robust protection against severe COVID-19 disease and hospitalization, at least in the U.S.
Officials at the FDA previously had expressed skepticism about the need for Pfizer COVID vaccine booster shots in a 23-page document released Sept. 16, prior to the meeting, on the agency’s website.
The report analyzed data submitted by Pfizer and BioNTech as part of the drugmakers’ request for authorization for their vaccine to be given as a booster shot in people 16 years and older. FDA officials said, based on their analysis of data submitted by Pfizer and BioNTech, they could not yet take a stance on whether to recommend COVID boosters for the general public.
16-year-old Sara Green “wants life back” after developing neurological problems following Pfizer vaccine
Sarah Green was a healthy 16-year-old until she developed neurological problems after receiving her second dose of Pfizer’s COVID vaccine. In an exclusive interview with The Defender, Sarah (VAERS I.D. 1354500) and her mother, Marie Green, said they feel helpless because nobody will acknowledge Sarah’s vaccine injury and “nobody can help them.”
Sarah received her second dose of Pfizer on May 4, and immediately began experiencing headaches. She then developed facial twitches and tremors, lost the ability to write, cannot drive and had to drop two college classes, Green said.
Sarah has seen numerous doctors who refuse to acknowledge the vaccine caused her condition. One doctor said Sarah had functional movement disorder and it was not related to the vaccine — although she said she has seen more cases since COVID vaccines were approved because people “stress themselves out over the vaccine and it’s psychosomatic.”
Green said she and Sarah are not anti-vaxxers, but there are too many people having problems for them not to know there’s a problem with mRNA vaccines.
Champion show jumper, 22, develops blood clots after Moderna COVID Vaccine
Imogen Allen, 22, developed two blood clots in her lungs after receiving Moderna’s COVID vaccine and will be on blood thinners for the rest of her life, the Daily Mail reported. Allen was diagnosed with a bilateral pulmonary thromboembolism after collapsing while on a family vacation two weeks after being vaccinated.
Allen was told by doctors the clots could have been triggered by the vaccine alongside five years on the contraceptive pill. Allen, a champion show jumper, may never be able to ride a horse again and her dreams of becoming a police detective were dashed after she was left bedbound.
“I was always wary of something happening, and it just shows that I had every right to be, because look at me now,” Allen said.
Babies could be given COVID vaccines in U.S. this winter
Pfizer’s COVID vaccine could be rolled out to babies as young as 6 months in the U.S. this winter — under plans being drawn up by the pharmaceutical giant.
According to the Daily Mail, Pfizer plans to apply for authorization to immunize American infants within the next two months, although the timeline will depend on findings of in-house trials that assess safety and efficacy of children aged six months to 5 years.
Frank D’Amelio, CFO and executive vice president of global supply at Pfizer, said in an industry conference last week the firm plans to “go file” by November, the Financial Times reported.
“We would expect to have … data for children between the ages of 6 months and 5 years old that we would file with the FDA,” D’Amelio said at the Morgan Stanley Global Healthcare Conference. “I’ll call it in the weeks shortly thereafter the filing of the data for the 5- to 11-year-olds.”
Pfizer plans to seek approval from the FDA for the shots to be given in children aged 5 to 11 by October.
Young boys at higher risk of hospitalization from Pfizer vaccine than from COVID
According to a new pre-print study, healthy boys between the ages of 12 and 15, with no underlying medical conditions, were four to six times more likely to be diagnosed with vaccine-related myocarditis than they were to be hospitalized with COVID.
To identify children with evidence of cardiac injury, researchers reviewed reports submitted to VAERS of adolescents between the ages of 12 and 17 who received an mRNA COVID vaccine.
The researchers identified a total of 257 cardiac adverse events (CAE) using the CDC’s working case definition of myocarditis, and found the post-vaccination CAE rate was highest in 12- to 15-year-old boys following their second dose of Pfizer. About 86% of the boys affected required hospital care, the authors said.
Dr. Tracy Høeg, physician, epidemiologist and associate researcher at UC Davis, found the rate of myocarditis after two doses of Pfizer’s vaccine to be 162.2 cases per million for healthy 12- to 15-year-old boys, and 94 cases per million for healthy 16- to 17-year-old boys. The equivalent rates for girls were 13.4 and 13 cases per million, respectively.
At current U.S. infection rates, the risk of a healthy adolescent being taken to the hospital with COVID in the next 120 days is about 44 per million, they said.
Experts accuse CDC of ‘cherry-picking’ data on natural immunity
There is a growing body of literature that shows natural immunity not only confers robust, durable and high-level protection against COVID, but also provides better protection than vaccine-induced immunity.
Yet, the CDC is ignoring the science of natural immunity when it comes to COVID, while acknowledging it for other diseases, said Dr. Marty Makary, professor of surgery and health policy at Johns Hopkins University. On Sept. 14, Makary said on the “Clay Travis and Buck Sexton Show,” the agency is providing contradictory, “illogical” COVID messaging. He accused the CDC of “cherry-picking” data and manipulating public health guidance surrounding vaccines and natural immunity to support a political narrative.
Makary explained how the CDC’s current guidance for chickenpox, for example, does not encourage those who have contracted it to vaccinate themselves against the virus. The CDC only recommends two doses of chickenpox vaccine for children, adolescents and adults who have never had chickenpox.
Makary called the conflicting guidance “absolutely illogical,” and accused the agency of “ignoring natural immunity.” He added the CDC is engaging in a statistical technique called “fishing,” where “you look for a tiny sliver of data that supports what you already believe.”
194 days and counting, CDC ignores The Defender’s inquiries
According to the CDC website, “the CDC follows up on any report of death to request additional information and learn more about what occurred and to determine whether the death was a result of the vaccine or unrelated.”
On March 8, The Defender contacted the CDC with a written list of questions about reported deaths and injuries related to COVID vaccines. We have made repeated attempts, by phone and email, to obtain a response to our questions.
Despite multiple phone and email communications with many people at the CDC, and despite being told that our request was in the system and that someone would respond, we have not yet received answers to any of the questions we submitted. It has been 194 days since we sent our first email to the CDC requesting information.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.