Experts Tell FDA Vaccines ‘Harm More People Than They Save,’ But NIH Director Believes Boosters Will Be Approved in Coming Weeks
During the Sept. 17 meeting of the FDA advisory panel to recommend whether to approve a third dose of Pfizer’s COVID vaccine, physicians pointed to data they said confirm the risks of Pfizer’s COVID vaccine don’t outweigh the benefits.
The Defender is experiencing censorship on many social channels. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. It's free.
National Institutes of Health (NIH) Director Dr. Francis Collins said he would be “surprised” if COVID booster shots were not recommended for other Americans in the upcoming weeks even after the U.S. Food and Drug Administration’s (FDA) advisory committee on Sept. 17 overwhelmingly rejected a proposal to distribute booster shots of Pfizer and BioNTech’s COVID vaccine to the general public.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) recommended the agency approve Pfizer’s application for boosters only for people 65 and older and certain high-risk populations.
In a conversation with “Fox News Sunday,” Collins dismissed the FDA’s decision as being subject to change upon further review of the science.
“I think the big news is that they did approve the initiation of boosters,” Collins said, for older and at-risk Americans. “Remember, they’re taking a snapshot of right now, we’re going to see what happens in the coming weeks.”
Collins said it would surprise him if it does not become clear over the next few weeks that the administration of boosters may need to be expanded. “Based on the data we’ve already seen both in the U.S. and in Israel, it’s clear that the waning effectiveness of those vaccines is a reality and we need to respond to it,” Collins said.
Collins said he was not sure whether boosters will be recommended for all — pointing to concerns of risks outweighing benefits for younger people — but he maintained that boosters for people under 65 will be approved.
Two FDA officials and a group of other leading scientists recently asserted that available evidence does not yet support encouraging COVID booster shots for all Americans.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to President Biden, said the FDA’s final decision on making booster shots available is expected later this week. The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices is separately expected to weigh in on Pfizer’s booster request.
Fauci on Sunday told ABC’s “This Week” that Biden planned to have booster shots ready as soon as this week, pending FDA approval, because “we wanted to be ready.”
“These are the kind of things that when you make a decision, you don’t snap your finger and it gets rolled out the next day,” Fauci said. “When the FDA makes their final determination and very soon thereafter this coming week, you’re going to see the Advisory Committee on Immunization Practices that advises the CDC to perhaps even fine-tune that, so it can be implemented expeditiously.”
Fauci said FDA decisions on booster shots for people vaccinated with Moderna’s or Johnson & Johnson’s vaccines are a few weeks away.
‘COVID Vaccines harm more people than they save,’ physicians tell FDA
During Friday’s meeting, VRBPAC unexpectedly voted against approving boosters for the general population based on a lack of long-term data and stating the risks did not outweigh the benefits.
During the public comment session, numerous experts said data supporting Pfizer’s request for booster doses was inadequate, and several highlighted concerning patterns with data from the CDC’s Vaccine Adverse Event Reporting System or VAERS — requesting more attention be given to potential signals and reported adverse events.
Dr. Jessica Rose, a viral immunologist and virologist stated she “took it upon herself to become a VAERS analyst who organizes data into comprehensive figures to convey information to the public in both published work and video medium.”
Rose said “safety and efficacy are the cornerstones of the development and administration of biological products meant for human use.” She provided a data bridge showing the probability of an adverse event occurring and the severity of the resulting harm to health of individuals in the design population.
“This is a barsoft that shows the past 10 years of VAERS data plotted against the total number of adverse event reports for all vaccines for the years 2011 to 2020 and for COVID associated products — only for 2021,” Rose said.
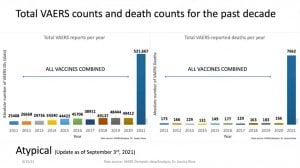
“The left barsoft represents all adverse event reports and the right barsoft represents all death adverse event reports,” Rose said. “There’s an over 1000% increase in the total number of adverse events for 2021, and we are not done with 2021. This is highly anomalous on both fronts.”
Rose said:
“The onus is on the public health officials at the FDA, the CDC and policymakers to answer to these anomalies and acknowledge the clear risk signals emerging from VAERS data and to confront the issue of COVID injectable products use/risk. In my opinion [the risks] outweigh any potential benefit associated with these products, especially for children.”
Rose also pointed out that as of Aug. 27, there were 1,500 adverse reactions occurring per million fully injected people, and 1 in 660 individuals are “succumbing to and reporting immunological adverse events associated with the COVID products.” Rose noted adverse events are under-reported and the under-reporting factor was not considered in her data.
Dr. Joseph Fraiman, an emergency medicine physician in New Orleans, revealed during his presentation to the FDA’s safety panel that no clinical evidence exists to disprove claims that the COVID vaccines are harming more people than they save.
Fraiman said he was there to ask for help to reduce vaccine hesitancy, however, in order to do this, large clinical trials that demonstrate vaccines reduce hospitalizations without finding evidence of serious harm are needed.
“I know many think the vaccine-hesitant are dumb or just misinformed, that’s not at all what I’ve seen,” Fraiman said. “In fact, typically, independent of education level, the vaccine-hesitant I’ve met in the ER are more familiar with vaccine studies and more aware of their COVID risks than the vaccinated.”
Fraiman said that without booster trials that are large enough to find a risk reduction in hospitalizations, “we, the medical establishment, cannot call out anti-COVID vaccine activists who publicly claim the vaccine harms more than they save, especially in the young and healthy. The fact that we do not have the clinical evidence to say these activists are wrong should terrify us all.”
Steve Kirsch, founder of the COVID-19 Early Treatment Fund, said he was going to focus on the elephant in the room that “nobody wants to talk about” — that COVID vaccines kill more people than they save.
He said:
“We were led to believe that vaccines are perfectly safe, but this is simply not true. For example, there were four times as many heart attacks in the treatment group in the Pfizer 6-month trial report. That wasn’t bad luck, the VAERS shows heart attacks happen 71 times more often following these vaccines compared to any other vaccine. In all, 20 people died who got the drug — 14 died who got the placebo.”
“If the net all cause mortality from the vaccines is negative, then vaccines, boosters and negatives are all nonsensical,” Kirsch said. “Even if the vaccines had 100% protection, it still means we kill two people to save one life.”
Kirsch said four experts did analyses using completely different non-U.S. data sources, and all of them came up with approximately the same number of excess vaccine-related deaths — about 411 deaths per million doses. “That translates into 150,000 people who have died [from COVID vaccines],” he explained.
Kirsch ended his presentation by discussing Maddie de Garay’s case. De Garay participated in Pfizer’s clinical trial when she was 12 years old and became paralyzed following her first COVID vaccine dose. Kirsch asked the panel why Pfizer didn’t report her injury in their results and wanted to know “why this fraud was not investigated.”
Kim Witczak, FDA consumer representative and founder of Woody Matters, a drug safety organization, said, “While boosters may be good for business mRNA vaccines were never designed to stop transmission or eradicate the virus.”
Witczak called out the government for not recognizing natural immunity for vaccine mandates and for the potential of “leaky vaccines” to produce variants.
Dr. Peter Doshi, professor at the University of Maryland and senior editor of The BMJ, asked the committee what problem a third dose is intended to solve. “If this is a pandemic of the unvaccinated, why would a fully vaccinated person need a third dose?” he asked.
Doshi said a third dose, fourth dose or fifth dose might nudge up antibodies, but what clinical difference does this make? It is vital to assess whether there’s a higher risk of harm associated with a third dose and to date, “we are still in the dark,” he said.
Doshi ended with an important question:
“Last week, three medical licensing boards said they could revoke doctors’ medical licenses for providing COVID vaccine misinformation. I’m worried about the chilling effects here. There are clearly many remaining unknowns and science is all about proving unknowns.
“But in the present supercharged climate — and I’ll point out that many members on this committee are certified by these boards — what is the FDA doing so that members can speak freely without fear of reprisal?”
FDA could choose to ignore its safety panel
As STAT reported, the FDA is not required to follow the recommendations of its advisory panel, though it generally does. But if the agency doesn’t, it will raise significant questions of political interference and will pit agency scientists against political officials who signed off on the booster plan.
In an unusual move last month, Biden and top health officials, including Surgeon General Vivek Murthy, acting FDA Commissioner Dr. Janet Woodcock and CDC Director Dr. Rochelle Walensky, publicly announced a booster shot program would begin the week of Sept. 20, well before the FDA and CDC examined the evidence.
Since then, numerous scientists have expressed skepticism over the need for COVID boosters, including two FDA officials who recently resigned over the issue.
On Thursday, FDA scientists had expressed skepticism about the need for Pfizer COVID vaccine booster shots in a 23-page report released Sept. 15 that called into question the limited data Pfizer had supporting its application for boosters.

