Side Effects and Data Gaps Raise Questions on COVID Vaccine
- January 26, 2021
Story at-a-glance
- Reports of serious side effects to the COVID-19 vaccines have started emerging. Examples include persistent malaise and extreme exhaustion, anaphylactic reactions, multisystem inflammatory syndrome, chronic seizures and convulsions, paralysis and sudden death within hours or days
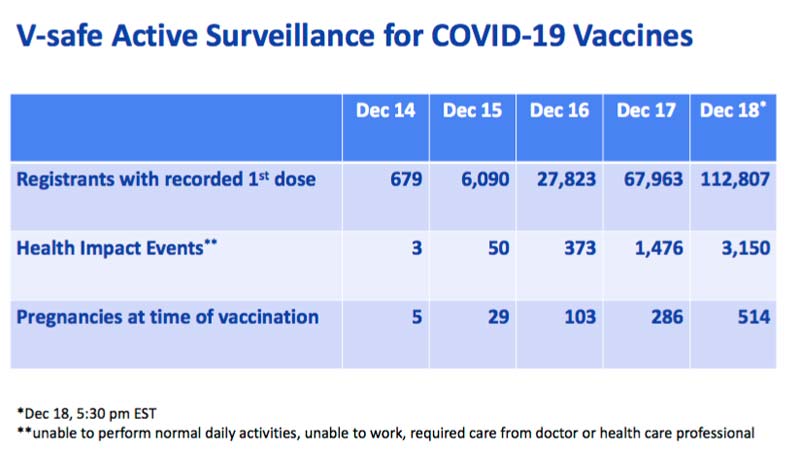
- By December 18, 2020, 112,807 Americans had received their first dose of COVID-19 vaccine. Of those, 3,150 suffered one or more “health impact events.” That’s a side effect rate of 2.79%
- While Pfizer claims its vaccine is 95% effective, this is the relative risk reduction. The absolute risk reduction is actually less than 1%
- Analysis of recently released data suggests the relative risk reduction for Pfizer’s vaccine may actually be between 19% and 29% — far lower than the required licensing threshold of 50%
- Studies have warned COVID-19 vaccines may result in more serious disease when exposed to the virus, either through antibody-dependent immune enhancement or pathogen priming that triggers an autoimmune response
While many have hitched their hope for a return to normalcy and a sense of safety to the rollout of COVID-19 vaccines, early reports are cause for concern. It didn't take long before reports of serious side effects started emerging in popular media and on social media networks. Examples include:
Persistent malaise1,2 and extreme exhaustion3 |
Anaphylactic reactions4,5,6 |
Multisystem inflammatory syndrome7 |
Chronic seizures and convulsions8,9,10 |
Paralysis,11 including cases of Bell's Palsy12 |
Sudden death within hours or days13,14,15 |
High Rate of Side Effects
According to the U.S. Centers for Disease Control and Prevention,16 by December 18, 2020, 112,807 Americans had received their first dose of COVID-19 vaccine. Of those, 3,150 suffered one or more "health impact events," defined as being "unable to perform normal daily activities, unable to work, required care from doctor or health care professional."
That gives us a side effect rate of 2.79%. Extrapolated to the total U.S. population of 328.2 million, we may then expect 9,156,780 Americans to be injured by the vaccine if every single man, woman and child is vaccinated.
When I checked the Vaccine Adverse Event Reporting System (VAERS) January 13, 2021, the total number of reported adverse events for the COVID-19 vaccine (any manufacturer) stood at 3,920.
Effectiveness Questioned
In a January 4, 2021, article17 in The BMJ Opinion, Peter Doshi, associate editor of The BMJ, again raised questions about the stated efficacy rate of Pfizer's and Moderna's COVID-19 vaccines, saying "we need more details and the raw data."
Previously, in a November 26, 2020, BMJ article,18 Doshi had pointed out that while Pfizer claims its vaccine is 95% effective, this is the relative risk reduction. The absolute risk reduction is actually less than 1%. This is the typical Big Pharma trick: confusing absolute and relative risks. They played this card in spades with the statin drugs and made tens if not hundreds of billions in profits. He also stressed that severe side effects appear commonplace:
"Moderna's press release states that 9% experienced grade 3 myalgia and 10% grade 3 fatigue; Pfizer's statement reported 3.8% experienced grade 3 fatigue and 2% grade 3 headache. Grade 3 adverse events are considered severe, defined as preventing daily activity. Mild and moderate severity reactions are bound to be far more common."
In his January 4 article,19 Doshi delves into recently released summary data20 given to the Food and Drug Administration. "While some of the additional details are reassuring, some are not," he says. In fact, his article outlines yet additional concerns "about the trustworthiness and meaningfulness of the reported efficacy results" of these two vaccines based on that data.
Relative Risk Reduction May Be Far Below Required Threshold
For starters, Doshi points out that Pfizer did not consistently confirm whether test subjects who showed symptoms of COVID-19 were actually PCR positive. Instead, a large portion of them were simply marked as "suspected COVID-19."
In all, there were 3,410 cases of "suspected but not confirmed" COVID-19 in the total study population (vaccine group and controls), 1,594 of which occurred in the vaccine group. Only eight cases in the vaccine group were actually confirmed with PCR testing. The problem with this is that the 95% effectiveness rating is based on PCR confirmed cases only. Doshi writes:21
"With 20 times more suspected than confirmed cases, this category of disease cannot be ignored simply because there was no positive PCR test result. Indeed, this makes it all the more urgent to understand.
A rough estimate of vaccine efficacy against developing COVID-19 symptoms, with or without a positive PCR test result, would be a relative risk reduction of 19% — far below the 50% effectiveness threshold for authorization set by regulators.
Even after removing cases occurring within 7 days of vaccination (409 on Pfizer's vaccine vs. 287 on placebo), which should include the majority of symptoms due to short-term vaccine reactogenicity, vaccine efficacy remains low: 29%."
It's worth noting that, for some reason, far more people in the vaccine group ended up with COVID-19 symptoms within that first week than did those in the placebo group.
Doshi goes on to state that if suspected cases occurred in people who had false negative PCR test results, then the vaccine's efficacy would be lowered even further. He also stresses that "average clinical severity" is not really all that important. What really matters is the "incidence of severe disease that affects hospital admissions."
Unfortunately, and this is really shocking, the trials were not designed to assess whether the vaccines prevent transmission of the infection. Since they don't, "an analysis of severe disease irrespective of etiologic agent — namely, rates of hospitalizations, ICU cases, and deaths amongst trial participants — seems warranted, and is the only way to assess the vaccines' real ability to take the edge off the pandemic," Doshi writes.
Why Were so Many in Vaccine Group Excluded?
Another concern brought forth in Doshi's article is the exclusion of 371 participants from Pfizer's efficacy analysis due to "important protocol deviations on or prior to seven days after Dose 2." Of those, 311 were from the vaccine group while only 60 were in the placebo group.
This marked imbalance is cause for concern. Why were five times as many in the vaccine group excluded from the efficacy analysis than in the placebo group? And what exactly were these "protocol deviations" that caused them to be excluded? This is called stacking the deck so the results can be manipulated in the desired direction to "prove" effectiveness, when it is merely a statistical manipulation.
Confounding Factors
Doshi is also concerned about the confounding role of pain and fever medications. These kinds of medications can mask symptoms, resulting in mild cases of COVID-19 going undetected, especially since all participants were not tested. They can also mask side effects of the vaccine.
The data suggest that in the Pfizer trial, pain and fever medication was taken three to four times more often by vaccine recipients than among those in the placebo group though, and according to Doshi:
"Their use was presumably concentrated in the first week after vaccine use, taken to relieve post-injection local and systemic adverse events. But the cumulative incidence curves suggest a fairly constant rate of confirmed COVID-19 cases over time, with symptom onset dates extending well beyond a week after dosing.
That said, the higher rate of medication use in the vaccine arm provides further reason to worry about unofficial unblinding. Given the vaccines' reactogenicity, it's hard to imagine participants and investigators could not make educated guesses about which group they were in. The primary endpoint in the trials is relatively subjective making unblinding an important concern."
He also questions Pfizer's use of an "adjudication committee" to count COVID-19 cases. "Were they blinded to antibody data and information on patients' symptoms in the first week after vaccination?" he asks.
"What criteria did they employ, and why, with a primary event consisting of a patient-reported outcome (COVID-19 symptoms) and PCR test result, was such a committee even necessary?" Furthermore, the committee consisted not of licensed doctors but of Pfizer staff members, which makes one wonder whether they had the appropriate qualifications to determine whether someone might have COVID-19 or not.
Does Vaccine Work in Those Who Already Had COVID-19?
Lastly, it's important to ascertain how the vaccine works for those who have already had COVID-19, seeing how the vaccine is recommended for everyone, regardless of whether or not you've already recovered from the infection. Here, the data reveal something rather odd. Doshi writes:22
"Individuals with a known history of SARS-CoV-2 infection … were excluded from Moderna's and Pfizer's trials. But still 1,125 (3.0%) and 675 (2.2%) of participants in Pfizer's and Moderna's trials, respectively, were deemed to be positive for SARS-CoV-2 at baseline …
By my count, Pfizer apparently reported 8 cases of confirmed, symptomatic COVID-19 in people positive for SARS-CoV-2 at baseline (1 in the vaccine group, 7 in the placebo group …) and Moderna, 1 case (placebo group …)
But with only around four to 31 reinfections documented globally, how, in trials of tens of thousands, with median follow-up of two months, could there be nine confirmed covid-19 cases among those with SARS-CoV-2 infection at baseline?
Is this representative of meaningful vaccine efficacy, as CDC seems to have endorsed? Or could it be something else, like prevention of COVID-19 symptoms, possibly by the vaccine or by the use of medicines which suppress symptoms, and nothing to do with reinfection?"
Vaccine Rollout Coincides With Outbreak
Whether or not the vaccine is helpful or harmful in people who either had COVID-19 before, or are currently positive for SARS-CoV-2 or ill with COVID-19 symptoms, is an important question now that these vaccines are being rolled out.
Case in point: In Auburn, New York, a COVID-19 outbreak began December 21, 2020, in a Cayuga County nursing home.23,24 Before this outbreak, no one in the nursing home had died from COVID-19.
The next day, December 22, they started vaccinating residents and staff. The first death was reported December 29, 2020. Between December 22, 2020, and January 9, 2021, 193 residents (80%) received the vaccine, as did 113 staff members.
As of January 9, 2021, 137 residents had been infected and 24 had died. Forty-seven staff members had also tested positive for SARS-CoV-2 and one was on life-support. Considering we're seeing cases in which healthy young and middle-aged individuals die within days of receiving the vaccine, it's not inconceivable that the vaccine might have something to do with this dramatic rise in deaths among the elderly. In fact, I'd expect it.
You can rest assured that the public health authorities and media will never report on these observations. Anything that conflicts with vaccine safety and effectiveness will be intentionally and universally buried. This is precisely their modus operandi of the past three decades. If anything, the suppression of the facts will only be amplified.
Vaccine May Trigger More Serious Illness
One of the original concerns with COVID-19 vaccines was the possibility of paradoxical immune enhancement or antibody-dependent enhancement. As discussed in my May 2020 interview with Robert Kennedy Jr., embedded above for your convenience, this is why previous coronavirus vaccines have failed.
As noted in the study,25 "Informed Consent Disclosure to Vaccine Trial Subjects of Risk of COVID-19 Vaccine Worsening Clinical Disease," published in the International Journal of Clinical Practice October 28, 2020, "COVID‐19 vaccines designed to elicit neutralizing antibodies may sensitize vaccine recipients to more severe disease than if they were not vaccinated."
"Vaccines for SARS, MERS and RSV have never been approved, and the data generated in the development and testing of these vaccines suggest a serious mechanistic concern:
Vaccines designed empirically using the traditional approach (consisting of the unmodified or minimally modified coronavirus viral spike to elicit neutralizing antibodies), be they composed of protein, viral vector, DNA or RNA and irrespective of delivery method, may worsen COVID‐19 disease via antibody‐dependent enhancement (ADE)," the paper states.26
"The specific and significant COVID‐19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent."
The 2003 review paper, "Antibody-Dependent Enhancement of Virus Infection and Disease," explains it this way:27
"In general, virus-specific antibodies are considered antiviral and play an important role in the control of virus infections in a number of ways. However, in some instances, the presence of specific antibodies can be beneficial to the virus. This activity is known as antibody-dependent enhancement (ADE) of virus infection.
The ADE of virus infection is a phenomenon in which virus-specific antibodies enhance the entry of virus, and in some cases the replication of virus, into monocytes/macrophages and granulocytic cells through interaction with Fc and/or complement receptors.
This phenomenon has been reported in vitro and in vivo for viruses representing numerous families and genera of public health and veterinary importance. These viruses share some common features such as preferential replication in macrophages, ability to establish persistence, and antigenic diversity. For some viruses, ADE of infection has become a great concern to disease control by vaccination."
Pathogenic Priming — Another Significant Risk
But that's not all. SARS-CoV-2 vaccines may also trigger autoimmune reactions through a process called "pathogenic priming." According to a 2020 paper28 in the Journal of Translational Autoimmunity, "Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity," noting that the same may apply post-vaccination.
As explained in this paper, all but one of SARS-CoV-2 immunogenic epitopes are similar to human proteins. Epitopes29 are sites on the virus that allow antibodies or cell receptors in your immune system to recognize it.
This is why epitopes are also referred to as "antigenic determinants," as they are the part that is recognized by an antibody, B-cell receptor or T-cell receptor. Most antigens — substances that bind specifically to an antibody or a T-cell receptor — have several different epitopes, which allow it to be recognized by several different antibodies.
According to the author, some epitopes can cause "autoimmunological pathogenic priming due to prior infection or following exposure to SARS-CoV-2 … following vaccination."
In other words, if you've had the infection once, and get reinfected (either by SARS-CoV-2 or a sufficiently similar coronavirus), the second bout has a great potential to be more severe than the first. Similarly, if you get vaccinated and are then infected with SARS-CoV-2, your infection may be more severe than had you not been vaccinated.
For this reason, "these epitopes should be excluded from vaccines under development to minimize autoimmunity due to risk of pathogenic priming," the paper warns. The abstract lays out the basics of the pathogenic priming process.30
Autoimmune Reactions Involved in Many Lethal COVID-19 Cases
According to the author, autopsies suggest many lethal COVID-19 cases were likely due to autoimmune reactions. A combination of genetic and environmental factors will influence a person's individual risk for this, just as genetic and environmental factors influence your risk of getting sick from the virus in the first place.
"Among coronaviruses, the spike surface glycoprotein is known to play a role in neuroimmunopathology. However, the SARS-CoV-2 virus has numerous other proteins and polyproteins, each which may serve as an antigen source during infection leading to autoimmunity," the author notes.
"Remarkably, over 1/3 (11/27) of the immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system … Mapping of the overall gene list … revealed that many functions of the human adaptive immune system might be impacted via autoimmunity against these proteins and their interactors …
These results could explain in part the high rates of serious illness associated with SARS-CoV-2. They could also explain the lengthy asymptomatic period prior to presentation of symptoms characteristic of COVID-19. SARS-CoV-2 could impair the immune response, at first, and then, over time, the immune system could begin to mount an attack on the myriad of proteins …
Unintended consequences of pathogenesis from vaccines are not new, nor are they unexpected. They are unanticipated only if those who develop them do not include available knowledge in their formulation plan.
For example, the H1N1 influenza vaccine used in Europe led to narcolepsy in some patients, resulting from homology between the human hypocretin (aka, orexin) receptor 2 molecule and proteins present in the vaccine. This was established via the detection of cross-reactive antibodies in the serum of patients who develop narcolepsy following H1N1 vaccination in Europe.
The fact that pathogenic priming may be occurring involving autoimmunity against multiple proteins following CoV vaccination is consistent with other observations observed during autoimmunity, including the release of proinflammatory cytokines and cytokine storm."
Do a Risk-Benefit Analysis Before Making Up Your Mind
Additional studies explaining how coronavirus vaccines can cause problems can be found in "How COVID-19 Vaccine Can Destroy Your Immune System." In closing, I would urge you to review the science before making up your mind about the vaccine. That includes mortality data for COVID-19, which is actually surprisingly low.
The lethality of COVID-19 is actually lower than the flu for those under the age of 60.31 If you're under the age of 40, your risk of dying from COVID-19 is just 0.01%, meaning you have a 99.99% chance of surviving the infection. And you could improve that to 99.999% if you're metabolically flexible, insulin sensitive, and vitamin D replete.
So, really, what are we protecting against with a COVID-19 vaccine? As mentioned, the vaccines aren't even designed to prevent infection, only reduce the severity of symptoms. Meanwhile, they could potentially make you sicker once you're exposed to the virus, and/or cause persistent serious side effects such as those listed at the beginning of this article.
While I won't tell anyone what to do, I would urge you to take the time to CAREFULLY review the science and weigh the potential risks and benefits based on your individual situation before you make a decision that you may regret for the rest of your life, which can actually be shortened with this vaccine.

No comments:
Post a Comment